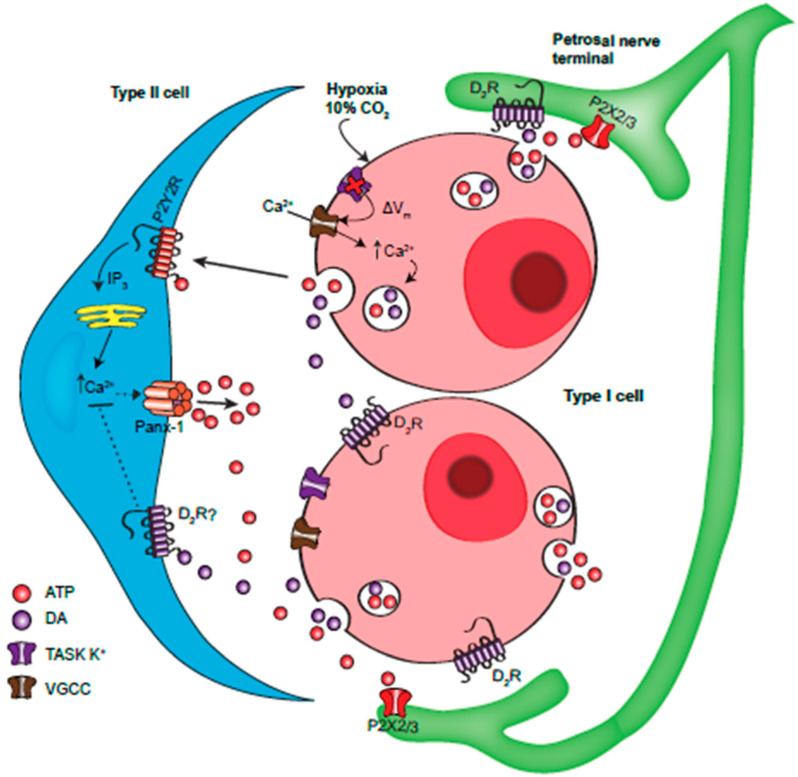
Figure 6.
Model of the proposed interactions involving ATP and dopamine at the carotid body ‘tripartite’ synapse. During hypoxia or hypercapnia type I cells depolarize due to inhibition of Two-pore domain, Acid-Sensitive K+ (TASK) channels, leading to membrane depolarization, Ca2+ entry through voltage-gated Ca2+ channels (VGCC) and vesicular release of ATP and DA. ATP activates postsynaptic P2X2/3R on petrosal afferent terminals causing excitation. ATP also activates P2Y2R on adjacent type II glial cells, causing a rise in intracellular Ca2+ via the PLC-IP3-Ca2+ pathway. This leads to Ca2+-dependent opening of pannexin (Panx)-1 channels which act as conduits for the release of ATP, thereby contributing directly to petrosal excitation. Co-released DA acts on autocrine-paracrine D2 receptors on the same or adjacent type I cells, leading to a negative feedback inhibition of neurotransmitter release. DA can also act on postsynaptic D2 receptors on petrosal terminals, leading to inhibition of action potential firing. DA may also activate sulpiride-sensitive (D2 and/or D3) receptors on type II cells, leading to a blunting of the ATP-P2Y2R -mediated rise in intracellular Ca2+ by an unknown mechanism. In this way, DA limits the ability of type II cells to contribute to the synaptic ATP pool via the mechanism of ‘ATP-induced ATP release’ involving Panx-1 channels. Omitted for clarity are paracrine signalling pathways involving other CB neurotransmitters including adenosine, generated in part from the breakdown of extracellular ATP.