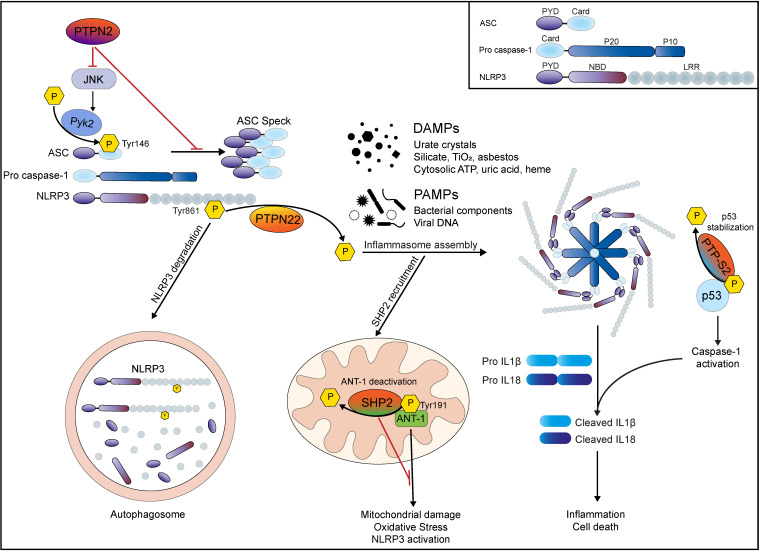
Figure 1.
Specific tyrosine phosphatases are involved in controlling inflammasome activation: PTPN22 regulates NLRP3 activation via direct dephosphorylation. Phosphorylated NLRP3 is recruited to autophagosomes for degradation, preventing excessive inflammasome activation. PAMP or DAMP stimulation promotes PTPN22 mediated NLRP3 dephosphorylation at Tyr861, leading to its activation. Subsequent assembly of the NLRP3 inflammasome complex results in activation of caspase-1 and consequent production of IL-1β and IL-18 and inflammatory cell death. PTPN2 negatively regulates inflammasome assembly via modulation of JNK and Pyk2 activity, inhibiting ASC Speck formation, and subsequent Caspase-1 activation. Upon inflammasome induction, tyrosine phosphatase SHP2 is recruited to mitochondria where it prevents mitochondrial damage via direct dephosphorylation of ANT-1 at Tyr191. This prevents a feed-forward amplifying loop of NLRP3 activation. Nuclear PTP-S2 dephosphorylates tumor suppressor gene p53 and thereby promotes its stability. Elevated levels of p53 promote pro-caspase-1 expression and accumulation, leading to elevated caspase-1 activation and subsequent IL-1β/IL-18 activation and inflammasome-associated cell death.