Figure 2.
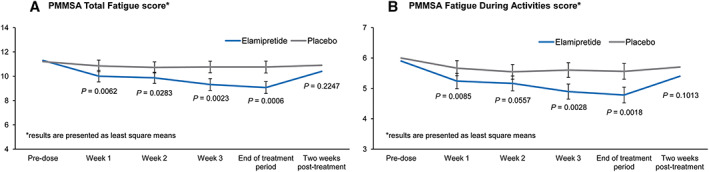
Primary Mitochondrial Myopathy Symptom Assessment (PMMSA) during the MMPOWER‐2 trial. Panel (A) shows the participants' total fatigue scores throughout the trial period. During the treatment with elamipretide, participants exhibited statistically significantly less total fatigue throughout the elamipretide treatment period (blue line) compared with the period while being treated with placebo (grey line) (95% CI, −2.6, −0.8; P = 0.0006). Panel (B) shows the participants' fatigue during activity scores throughout the trial period. During treatment with elamipretide, participants exhibited statistically significantly less fatigue during activities throughout the treatment period (blue line) compared with the period while being treated with placebo (grey line) (95% CI, −1.2, −0.3; P = 0.0018). For both scores, there was a steady improvement throughout the treatment period and a return to baseline score upon discontinuation of elamipretide therapy.
