Figure 5.
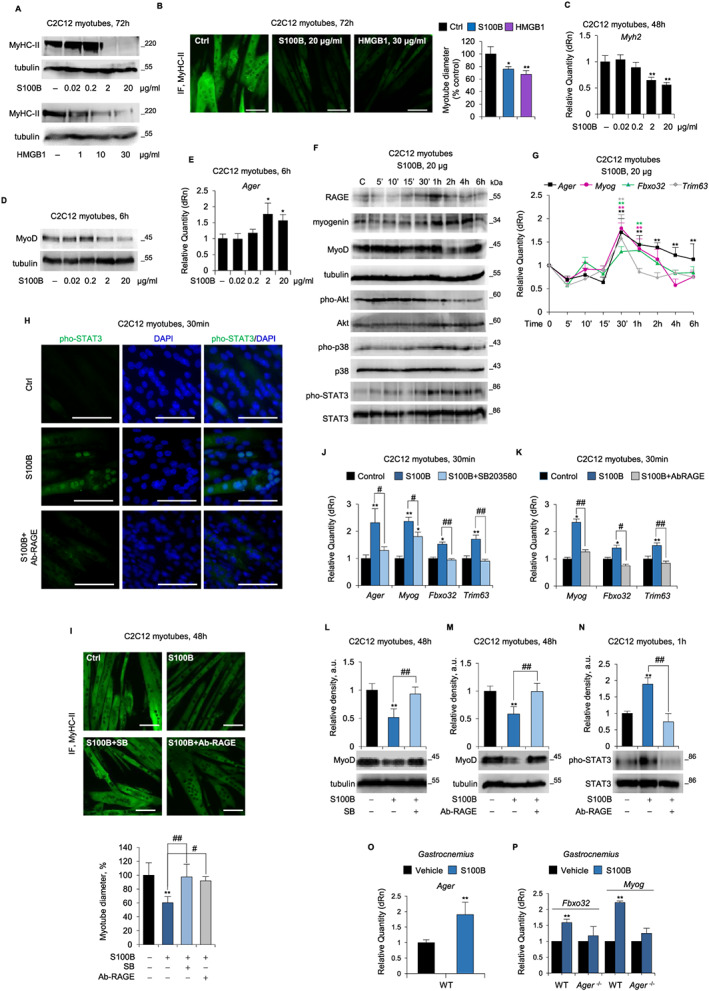
Dual trophic and atrophying effect of receptor for advanced glycation end‐products (RAGE) signalling in myotubes in the absence of cytokines. (A–N) C2C12 myotubes were cultured with S100 calcium‐binding protein B (S100B) (0–20 μg/ml) in the absence or presence of either Ab‐RAGE or SB203580, or with high mobility group box 1 (HMGB1) (0–30 μg/ml). Levels of MyHC‐II was evaluated by western blot (WB) (A), immunofluorescence (IF) (B) and real‐time PCR (C). Reported are the percentages of myotubes diameters relative to control (B). (D,E) myoblast determination protein 1 (MyoD) (D) and Ager (E) expression was analysed by western blot (WB) and real‐time PCR, respectively. (F) Levels of RAGE, myogenin, MyoD, and total and phosphorylated protein kinase B (Akt), p38 mitogen‐activated protein kinase (MAPK), and signal transducer and activator of transcription 3 (STAT3) were evaluated by WB. (G) Levels of Ager, Myog, Fbxo32, and Trim63 were analysed by real‐time PCR. (H) the localization of pho‐STAT3 was evaluated by IF. (I) The expression of myosin heavy chain (MyHC)‐II was evaluated by WB, and the percentage of myotubes diameters respect to control was determined. (J,K) Levels of Ager (J) and Myog, Fbxo32, and Trim63 (J,K) were analysed by real‐time PCR. (L‐N) Levels of MyoD protein (L,M) were analysed by WB, and total and phosphorylated STAT3 levels were analysed by WB (N). (O,P) Gastrocnemius muscles of WT and Ager −/− mice (n = 5 each group) were injected daily for three consecutive days with S100B (50 ng/muscle) or vehicle [phosphate‐buffered saline(PBS)] and analysed for the expression of Ager (O), and Fbxo32 and Myog (P) by real‐time PCR. Results are means ± standard error of the mean (B,I) or standard deviation (C,E,G,J–P). Statistical analysis was conducted using the two‐tailed t‐test. * P < 0.05 and ** P < 0.01, significantly different from internal control. # P < 0.05 and ## P < 0.01, significantly different. Scale bars (B,H,I), 100 μm.
