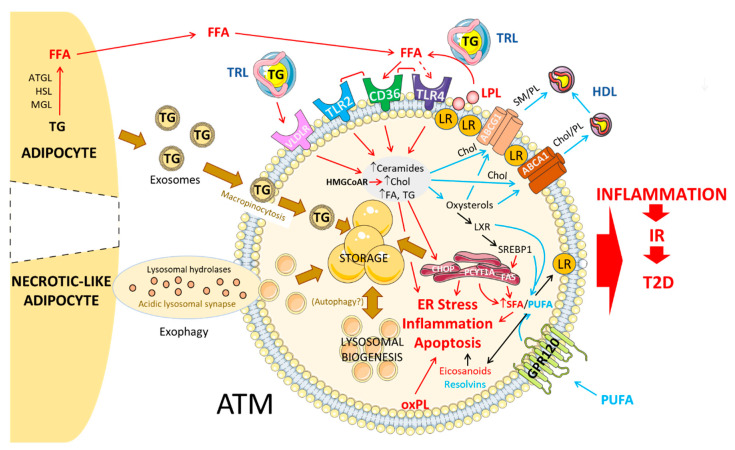
Figure 1.
Lipid handling in adipose tissue macrophages in diet-induced obesity.In diet-induced obesity, adipose tissue macrophages are metabolically activated by free fatty acids (FFA) released by lipolysis of triglycerides from adipocytes or from triglyceride-rich lipoproteins by lipoprotein lipase, through Cd36 and toll-like receptors. Accumulation and storage of lipids in adipose tissue macrophages (ATMs) under the form of lipid droplets occur through the uptake of triglyceride-rich lipoproteins by specific receptors such as very low-density lipoprotein (VLDLR) or through the internalization of adipocyte-derived exosomes by macropinocytosis. In crown-like structures, necrotic-like adipocyte clearance by ATM in acidic lysosomal synapses through a mechanism of exophagy also contributes to foam cell formation. As a result, ATMs are characterized by an increased content of triglycerides, free cholesterol, ceramides and fatty acids, which can either be synthesized into other lipids (See Figure 2), be stored in lipid droplets or catabolized through the lysosomal pathway. The accumulation of free cholesterol and sphingolipids such as sphingomyelin into membrane lipid rafts promotes the recruitment of TLR4 at the cell surface and contributes to the activation of inflammatory signaling pathways. ABCA1 and ABCG1 transporters promote the efflux of lipids from ATM to high-density lipoproteins allowing the reduction in lipid raft formation, thus ensuring an optimal lipoprotein lipase activity which participates in foam-like cell formation. Fatty acid synthesis, elongation and desaturation are under the control of liver X receptor (LXR) and sterol regulatory element-binding protein (SREBP1) thus exerting an important role in the relative cellular content of saturated and polyunsaturated fatty acids. Increased cellular levels of free cholesterol and saturated fatty acids trigger endoplasmic reticulum stress, inflammation and apoptosis, which can be alleviated by an enrichment in polyunsaturated fatty acids through mechanisms dependent or independent of the GPR120 receptor. Production of eicosanoids and resolvins from ω-6 and ω-3 polyunsaturated fatty acids, respectively, induces or represses inflammation in ATM. Increased turnover of phosphatidylcholine as well as oxidation of phospholipids also participate in the inflammatory status of ATM. As a whole, the rewiring of the lipid metabolism in ATM promotes adipose tissue inflammation and contributes to the establishment of insulin resistance and type 2 diabetes. ABCA1, ATP-binding cassette A1; ABCG1, ATP-binding cassette G1; ATGL, Adipose triglyceride lipase; Chol, cholesterol; CHOP, CCAAT-enhancer-binding protein homologous protein; FAS, fatty acid synthase; FFA, free fatty acids; GPR120, G protein-coupled receptor 120; HMGCoAR, 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase; HSL, hormone-sensitive lipase; IR, insulin resistance; LPL, lipoprotein lipase; LR, lipid rafts; LXR, liver X receptor; MGL, monoacylglycerol lipase; oxPL, oxidized PL; PCYT1A, phosphate cytidylyltransferase 1; PL, phospholipid; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid; SM, sphingomyelin; SREBP1, sterol regulatory element-binding protein 1; T2D, type 2 diabetes; TLR, toll-like receptor; TG, triglyceride; TRL, triglyceride-rich lipoproteins; VLDLR, very low-density lipoprotein receptor.