Fig. 4.
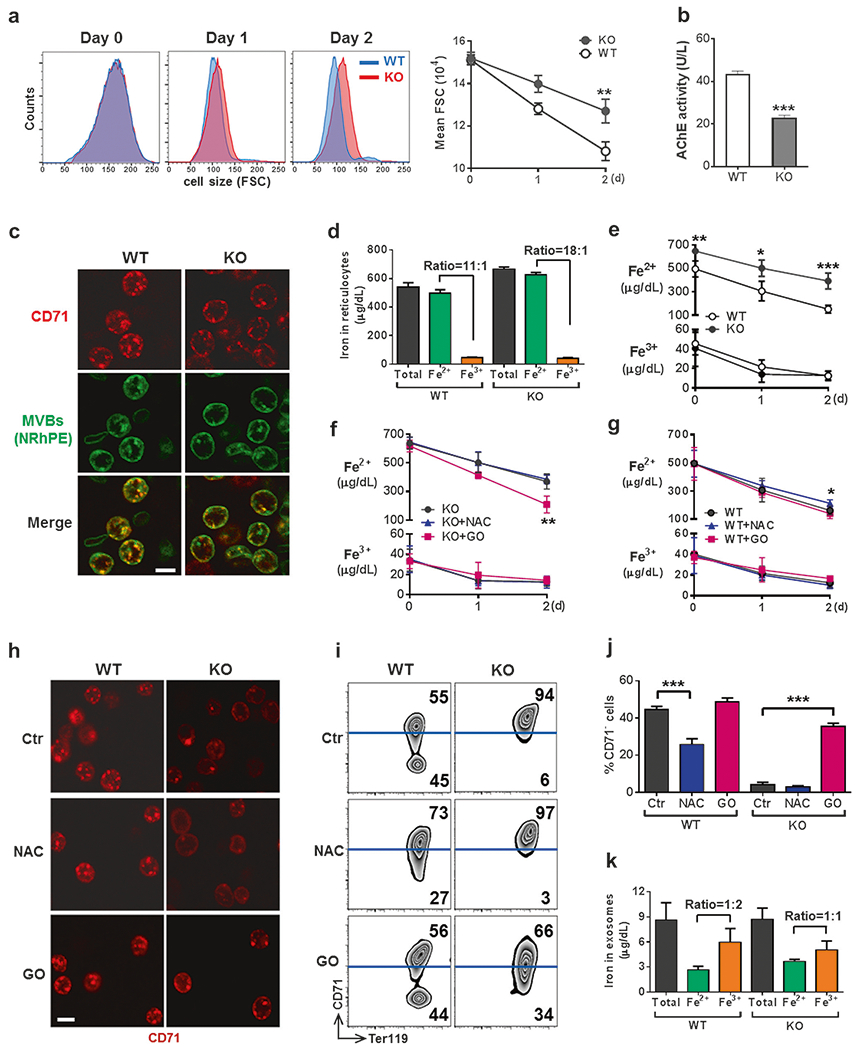
CD71 shedding is coupled with mitochondrial clearance in a ROS-dependent manner. a–c Exosome release is impaired in IEX-1 KO reticulocytes. Ter119+CD71high reticulocytes were sorted from PHZ-treated mice as above and cultured for 2 days. The size of reticulocytes was analyzed daily by flow cytometry on the basis of forward scatter (a). Exosomes released from 1 × 107 reticulocytes were assessed by the activity of AChE (b). After culturing for 12 h, cells were stained with FITC-anti-CD71 and N-Rh-PE that labeled MVBs to demonstrate their co-localization (c). Scale bar, 5 μm. Images were representative for at least 9 samples per group. d–g Changes of cellular iron concentrations during reticulocyte maturation. Concentrations of total iron, Fe2+, and Fe3+ were measured in reticulocytes before culturing (d) and daily during ex vivo maturation (e). f–i Modulation of ROS regulates CD71 shedding via exosome release. Ter119+CD71high reticulocytes as in a were cultured in the presence of 5 μM antioxidant NAC or 5 mU/mL glucose oxidase (GO), followed by daily measurement of cellular iron concentrations (f, g). Also shown are anti-CD71 staining for visualizing MVBs at 12 h after initial culture (h; scale bar, 5 μm), Ter119 and CD71 expression profiles by flow cytometry (i) with statistical analysis (j) on day 2, and concentrations of indicated irons and ratios of Fe2+ to Fe3+ measured in exosomes released from WT and KO reticulocytes 2 days after ex vivo maturation (k). Data represent as mean ± SEM (n = 12), *P < 0.05, **P < 0.01 and ***P < 0.001 compared between WT and KO cells or between indicated groups
