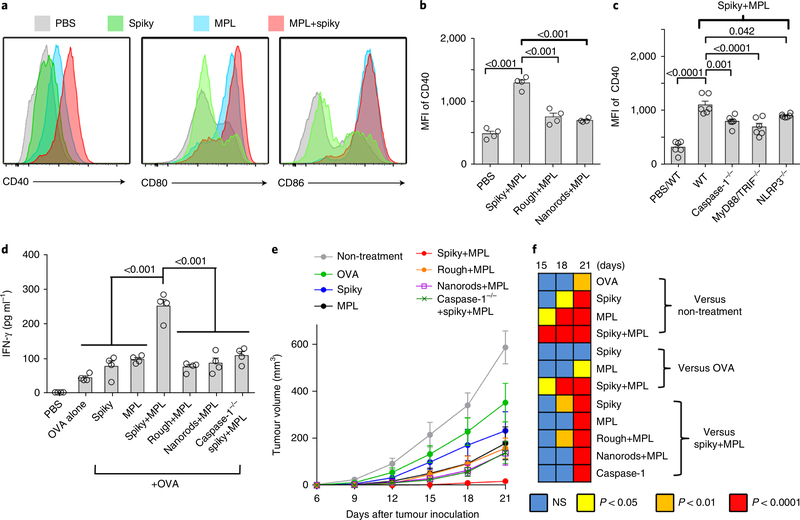
Fig. 5 |. Spiky particles enhance DC maturation and DC-meditated cancer immunotherapy.
a, Upregulation of the activation markers CD40, CD80 and CD86 on DCs. BMDCs were incubated with spiky particles, MPL or a combination of both for 12h. b, BMDCs were incubated with MPL plus spiky particles, rough particles or nanorods for 12 h, and the expression of the CD40 marker was analysed by flow cytometry. n = 4 biologically independent samples. MFI, mean fluorescence intensity. Representative flow cytometric profiles are given in Supplementary Fig. 14a. c, BMDCs from WT, caspase-1−/−, MyD88/ TRIF−/− and NLRP3−/− mice were cultured with spiky+MPL, and the expression of the CD40 marker was analysed by flow cytometry as in Supplementary Fig. 14b. n = 6 biologically independent samples. The particle dose was 0.04 particles μm−2 in b and c. d, In vitro CD8 T-cell activation assay. BMDCs from WT or caspase-1−/− mice were stimulated with antigen alone (OVA plus SIINFEKL peptide) or coupled with spiky particles, MPL alone or combinations. Activated DCs were subsequently incubated with T cells from OT-I mice for 3 d. The IFN-γ secreted from the T cells was measured by ELISA. n = 4 biologically independent samples. e, DC-mediated cancer immunotherapy. Mice were subcutaneously injected with 5×105 EG7 tumour cells and BMDCs, as in d, were administered 3 and 7d later. The tumour volumes were monitored every 3d. n = 8 tumours. f, The significance between the indicated groups on days 15–21 was calculated by two-way ANOVA and is shown as a heat map. All the experiments were repeated twice with similar results. The significance between the indicated groups in b-d was calculated by one-way ANOVA. Data are presented as the mean± s.e.m. NS, not significant.