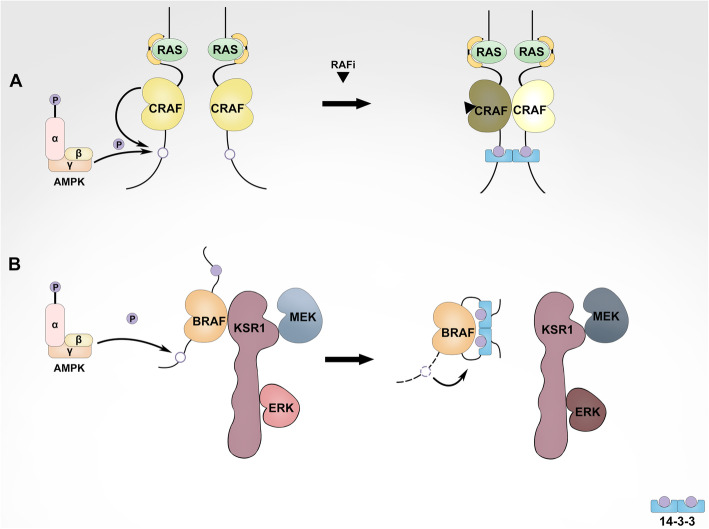
Fig. 4.
AMPK regulates differentially hyperactive Ras/RAF/MEK/ERK (MAPK) signaling in Ras- versus BRAF(V600E)-mutated cancers. a In Ras-mutated cancers, the C-terminal 14-3-3 binding site of CRAF is phosphorylated by AMPK, which facilitates CRAF dimerization through improving the association of CRAF dimer with 14-3-3 dimer and thus elevates the activity of CRAF, particularly upon RAF inhibitor treatment or metabolic stress. Under these conditions, CRAF forms homodimers with itself or heterodimers with KSR or BRAF. b In BRAF(V600E)-harboring cancers, AMPK phosphorylates the C-terminal 14-3-3 binding site of BRAF(V600E), which prevents BRAF(V600E) dimerization with KSR through enhancing the association of a single BRAF(V600E) molecule with 14-3-3 dimer and thus blocks the activity of BRAF(V600E) upon metabolic stress