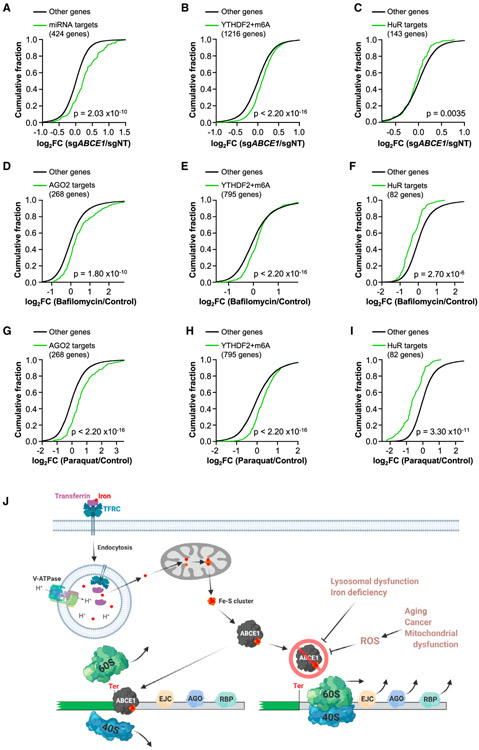
Figure 7. Depletion of ABCE1 Broadly Impairs 3′ UTR-Directed Regulation.
(A–C) CDF plots showing the effects of ABCE1 deficiency on the expression of miRNA targets, defined as mRNAs with FC > 1.5 in AGO2−/− HCT116 cells compared with wild-type cells (Golden et al., 2017) (A), mRNAs with m6A modification that were bound by YTHDF2 (Wang et al., 2014) (B), or HuR targets defined as the 150 most downregulated HuR-bound mRNAs in HuR-deficient cells (Lebedeva et al., 2011) (143 were expressed in HCT116 cells; C).
(D–I) CDF plots showing the effect of bafilomycin treatment (5 nM, D–F) or paraquat treatment (200 μg/mL, G–I) on the targets of post-transcriptional regulators shown in (A–C). Targets in each plot were restricted to those that were increased (AGO2 and YTHDF2 targets) or decreased (HuR targets) in ABCE1 knockout cells to specifically examine transcripts whose levels are regulated by ribosome recycling.
(J) ABCE1-mediated ribosome recycling links lysosomal dysfunction, iron-deficiency, and oxidative stress to 3′ UTR-directed post-transcriptional regulation and NMD. Lysosomal acidification is necessary for TF-mediated iron uptake, which is required for biogenesis of the Fe-S cluster that serves as a critical co-factor for ABCE1. Impaired ABCE1 function, occurring in the setting of reduced iron availability or elevated ROS, leads to movement of unrecycled ribosomes into 3′ UTRs, where they displace EJCs and other regulatory RNPs, disabling NMD and other forms of post-transcriptional regulation.
The p values for CDF plots were calculated by one-sides Kolmogorov-Smirnov test. See also Figure S7.