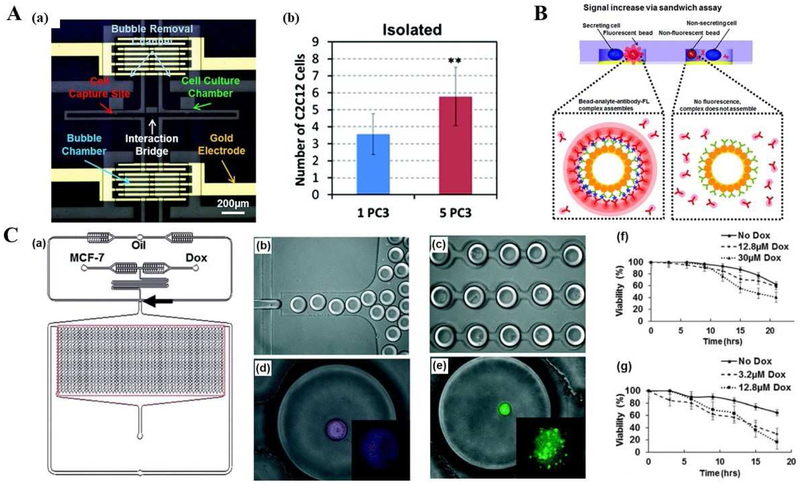
Fig. 4.
Single cell analysis applications using microfluidic platforms in cancer biology, diagnosis, and therapy. (A) Cell-cell interaction assay using a microfluidic platform. (a) Microphotograph of the fabricated device including culture chambers, an interaction bridge, bubble chambers, bubble removal channels, and a gold electrode for electrolysis. (b) Proliferation rates of C2C12 cells co-cultured with one PC3 and five PC3 cells with bubble isolation. Adapted with permission from Ref. [114]. Copyright 2014 Royal Society of Chemistry. (B) Principle of the reconfigurable microfluidic device for real-time monitoring of secreted proteins at the single-cell level. Each individual compartment consists of single cells and sensing beads surrounded by PE-labelled detection antibodies as reporter molecules. Once released by cells, target molecules diffused inside the chamber, bound to sensing bead, causing fluorescence to increase on the bead surface via a sandwich assay. Adapted with permission from Ref. [115]. Copyright 2016 The Royal Society of Chemistry. (C) Droplet array design, single cell encapsulation images and Dox cell viability tests with different MCF-7 cell lines. (a) Schematics of integrated microfluidic platform and droplet generation array. (b) Droplet generation. (c) Droplet docking in a microarray. (d) Image of live MCF-7S cell in a droplet after incubation with Cy-5 conjugated ABCB-1 mRNA. (e) Image of live Dox-resistant MCF-7R cell encapsulated in a droplet, Calcein AM was hydrolyzed after entering live cells. (f) Cumulative cell viability of MCF7 Dox-resistant (MCF-7R) cells (f) and MCF7 Dox-sensitive (MCF-7S) cells (g) treated with two concentrations of Dox. Adapted with permission from Ref. [116]. Copyright 2015 The Royal Society of Chemistry.