Abstract
Background
Myocardial infarction (MI) in elderly patients is associated with unfavorable prognosis, and it is becoming an increasingly prevalent condition. The prognosis of elderly patients is equally impaired in ST-segment elevation (STE) or non-STE (NSTE), and it is markedly worsened by the common presence of multivessel disease (MVD). Given the limited evidence available for elderly patients, it has not yet been established whether, as for younger patients, a complete revascularization strategy in MI patients with MVD should be advocated. We present the design of a dedicated study that will address this research gap.
Methods and design
The FIRE trial is a prospective, randomized, international, multicenter, open-label study with blinded adjudicated evaluation of outcomes. Patients aged 75 years and older, with MI (either STE or NSTE), MVD at coronary artery angiography, and a clear culprit lesion will be randomized to culprit-only treatment or to physiology-guided complete revascularization. The primary end point will be the patient-oriented composite end point of all-cause death, any MI, any stroke, and any revascularization at 1 year. The key secondary end point will be the composite of cardiovascular death and MI. Quality of life and physical performance will be evaluated as well. All components of the primary and key secondary outcome will be tested also at 3 and 5 years. The sample size for the study is 1,400 patients.
Implications
The FIRE trial will provide evidence on whether a specific revascularization strategy should be applied to elderly patients presenting MI and MVD to improve their clinical outcomes.
Myocardial infarction in elderly adults
As a result of shifting demographics and increased life expectancy, it is estimated that the number of elderly adults will increase by 44% from 2017 to 2030.1 Because the prevalence of coronary artery disease (CAD) is linked to aging, health care systems and professionals will have to deal with an increasing number of old patients presenting with acute and chronic presentation of ischemic heart disease. It is estimated that 15%-20% of subjects >75 years old develop MI.2 The challenge of dealing with an increasing number of elderly patients with MI goes beyond a mere statistical increase. Although cardiovascular (CV) death is slowly declining in the general population, it remains unchanged in elderly MI patients because they2 , 3 , 32are at higher risk of both ischemic and bleeding complications.2 , 4., 5., 6. Advanced age is associated with functional impairment and comorbidities, resulting in a more severe clinical presentation and complications due to invasive procedures and medical treatments and consequent worsening prognosis2 , 4., 5., 6.In addition, elderly patients with acute coronary syndrome (ACS) usually receive optimal medical therapy (OMT) but are more conservatively treated in terms of coronary artery angiography (CAA) and are the ones with the worst prognosis.7
Multivessel disease in elderly patients
Multivessel disease (MVD) is associated with a worse outcome in patients with CAD. Elderly patients undergoing CAA present MVD in 55% of the cases.2 , 8 In the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications trial, the mean number of diseased vessels increased with age and was related to a worsening prognosis.9 By multivariable analysis, the presence of triple-vessel disease was the strongest predictor of 1-year death (hazard ratio [HR] = 2.60, P = .009), death and reinfarction (HR = 1.88, P = .03), and major adverse cardiac events (HR = 1.80, P = .0009).9 Comparable results were found in the Western Denmark Heart Registry.10 In the Prospective Randomized Comparison of the BioFreedom Biolimus A9 Drug Coated Stent Versus the Gazelle Bare Metal Stent in Patients With High Risk of Bleeding trial, MVD was reported in 62% of patients, and it was the most important correlate for the 2-year ischemic end point (HR 1.66, 95% CI 1.27-2.18, P < .001).4 However, only 22% of patients received a multivessel revascularization.
Current treatment of elderly patients with ST segment elevation myocardial infarction and MVD
Reperfusion of the culprit lesion through primary percutaneous coronary intervention (PCI) is the standard of care in ST segment elevation myocardial infarction (STEMI) patients, regardless of their age.11 Registry data show that 51% of elderly STEMI patients present MVD and the majority of them (54%) receive culprit-only lesion treatment, whereas complete revascularization is achieved in only 31% of MVD patients.8 The management of nonculprit lesions in STEMI patients with MVD has been the focus of several randomized clinical trials (RCTs) comparing culprit-only versus complete revascularization strategies.12., 13., 14., 15., 16. The results of the largest RCT on the topic have been recently published.17 The Complete vs Culprit-Only Revascularization to Treat Multivessel Disease After Primary PCI for STEMI (COMPLETE) trial randomized 4,041 patients with STEMI and MVD.17 The main finding was the highly significant reduction of new MI occurrence in the complete group (7.9% vs 5.4%, HR 0.68, 95% CI 0.53-0.87, P = .002). Although the COMPLETE trial results will have an impact on the management of STEMI patients with MVD, their applicability to elderly MI patients is at least questionable. The mean age of patients enrolled in the COMPLETE trial was 62 ± 11 years. Patients ≥75 years old were underrepresented, like in all the other RCTs evaluating the best treatment strategy for MVD (Table I ). The rate of adverse events was extremely low, with CV death being around 3% at a median follow-up of 3 years. In addition, a recent subanalysis of the DANAMI-PRIMULTI-3 trial questioned the “one size fits all” approach in terms of revascularization strategy in STEMI patients with MVD, showing that in patients ≥75 years randomized to culprit-only or fractional flow reserve (FFR)–guided complete revascularization, there were no significant differences in the incidence of the primary end point (9 [15%] vs 11 [22%]; HR 1.49 [95% CI 0.57-4.65]; log-rank P = .19; P for interaction versus patients <75 years < .001).18 Besides, less than 30% of elderly ACS patients present ST-segment elevation at hospital admission.2
Table I.
Mean age and 1-year outcomes in contemporary trials focusing on revascularization strategy of STEMI patients with MVD
| Trial | Pts | Groups | Age (mean ± SD) | Outcome in the culprit-only arm |
||
|---|---|---|---|---|---|---|
| MI | Revascularization | MACE | ||||
| COMPLETE17 | 4041 | Angio/FFR complete vs culprit only | 62 ± 11 | 7.9%⁎ | 7.9%⁎ | 16.7%⁎ |
| COMPARE-ACUTE15 | 885 | FFR complete vs culprit only | 62 ± 10 | 4.7% | 17.5% | 20.5% |
| CvLPRIT13 | 296 | Angio-complete vs culprit only | 65 ± 12 | 2.7% | 8.2% | 21.2% |
| DANAMI-3 PRIMULTI14 | 627 | FFR complete vs culprit only | 64 ± 10 | 5% | 9% | 22% |
| Politi et al16 | 214 | Angio-complete vs culprit only | 65 ± 12 | 8.3% | 33.3% | 50% |
| PRAMI12 | 465 | Angio-complete vs culprit only | 62 ± 10 | 8.6% | 19.9% | 22.9% |
| Dambrink et al51 | 121 | FFR complete vs culprit only | 62 ± 10 | 0%† | 22%† | 22%† |
| Hamza et al52 | 100 | Angio-complete vs culprit only | 54 ± 11 | 4%† | 12%† | 24%† |
| Di Mario et al53 | 69 | Angio-complete vs culprit only | 64 ± 10 | 6% | 35% | 35% |
Pts, number of patients.
At 3 years.
At 6 months.
Current treatment of elderly patients with non–ST segment elevation myocardial infarction and MVD
Non–ST segment elevation myocardial infarction (NSTEMI) is considered a heterogeneous disease, and for this reason, it is more complex to approach in the design of RCTs. Consequently, all the studies focusing on a strategy to pursue in MVD in an ACS setting have been conducted only in a STEMI setting. However, NSTEMI is the most frequent clinical presentation in elderly patients.2 , 3 , 19., 20., 21. The management of STEMI and NSTEMI patients in terms of interventional and pharmacological strategies is similar.22 , 23 In particular, because the pathophysiology is common, namely, the disruption of a vulnerable atherosclerotic plaque or erosion of the coronary artery endothelium, the main goal is in both settings is to restore flow or avoid occlusion of the culprit vessel, although with different time frames.22 , 23 In addition, mortality in elderly patients after NSTEMI seems to be higher than the one after STEMI.24 Therefore, NSTEMI patients were included in a trial focused on identifying which revascularization strategy to pursue in elderly patients. In elderly NSTEMI patients, not only is the management of MVD unclear, but even the need for an invasive treatment is object of an ongoing RCT (NCT03052036). In the French Registry of Acute ST-Elevation or Non–ST-Elevation Myocardial Infarction registry, 76% of the patients not receiving CAA were aged ≥75 years.25
For what concerns the management of MVD, data are lacking. In the French Registry of Acute ST-Elevation or Non–ST-Elevation Myocardial Infarction registry, 5-year all-cause mortality in patients with NSTEMI medically managed and with MVD was 54.2%.25 In a large observational study, 58% of NSTEMI patients had MVD and only 54% received complete revascularization.26 The percentage of patients who had MVD increased over time, from 26% in 2005 to 36% in 2015. In this analysis, patients receiving complete revascularization presented reduced long-term mortality compared to those receiving culprit-only revascularization. However, a detailed meta-analysis of observational studies on multi- versus single-vessel PCI in Non-ST-segment elevation acute coronary syndrome (NSTEACS) (117,685 patients) showed no benefits in terms of either mortality (HR 0.79; 95% CI 0.58-1.09; I 2 67.9%) or the composite of death or MI (HR 0.90; 95% CI 0.69-1.17; I 2 62.3%), although both end points showed moderate inconsistency and evidence of publication bias (Egger test P = .097).27 There is no randomized comparison between culprit-only and complete revascularization in NSTEMI with MVD with hard clinical end point to date. A recent Scientific Statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society pointed out that additional studies are needed to define the risks and benefits of conservative versus invasive care in elderly patients with ACS.28
Treatment of MVD in elderly MI patients: the rationale for a physiology-guided approach
In most RCTs investigating the benefit of complete revascularization, the decision to proceed or not with revascularization was angiography guided. In the recent COMPLETE trial, the use of intracoronary physiology was negligible (<1%).17
There are several reasons to use physiology in elderly MI patients. First, functional guided revascularization can have the same benefit of angioguided revascularization in terms of MI reduction, and at the same time, the number of treated vessels is significantly reduced.14 , 15 , 29 , 30 Second, the presence of residual “anatomic” CAD after functionally complete revascularization has no impact on prognosis.31 Third, the occurrence of procedural complications, especially those impacting the prognosis such as periprocedural MI and Contrast Induced-Acute Kidney Injury (CI-AKI), is directly proportional to both age and number of treated vessels.32., 33., 34., 35., 36., 37. Fourth, periprocedural complications are associated with a worse prognostic impact in elderly patients.32., 33., 34., 35., 36., 37. Finally, the number of treated vessels is a major driver of prolonged dual antiplatelet therapy (DAPT) duration, which is associated with major bleeding and all-cause mortality in elderly patients.4 , 6
Rationale of the Functional versus Culprit-only Revascularization in Elderly Patients with Myocardial Infarction and Multivessel Disease trial
Elderly patients with MI and MVD have the worst prognosis among CAD patients. No trial has ever been designed to optimize their strategy and consequently their outcome. The main presentation of elderly MI patients is as NSTEMI, and their current real-life standard of care is, at best, the culprit-only revascularization. However, real-life registries show that their outcome is far from being optimal.24 To date, studies on elderly patients have been focused on devices (bare metal vs drug-eluting stent).4 , 5 , 38 , 39 In NSTEMI elderly patients, an ongoing trial is sought to understand what is better between a “selective” or a “routinely” invasive approach. However, when in elderly MI patients an invasive approach is selected and MVD is detected, there are no RCTs showing what is the best strategy to improve the prognosis. Our hypothesis is that functionally guided complete revascularization in elderly patients with MI and MVD, compared to the culprit-only revascularization, may reduce the occurrence of the composite patient-oriented end point of all-cause death, MI, stroke, and ischemia-driven revascularization.
Methods
Study design and population
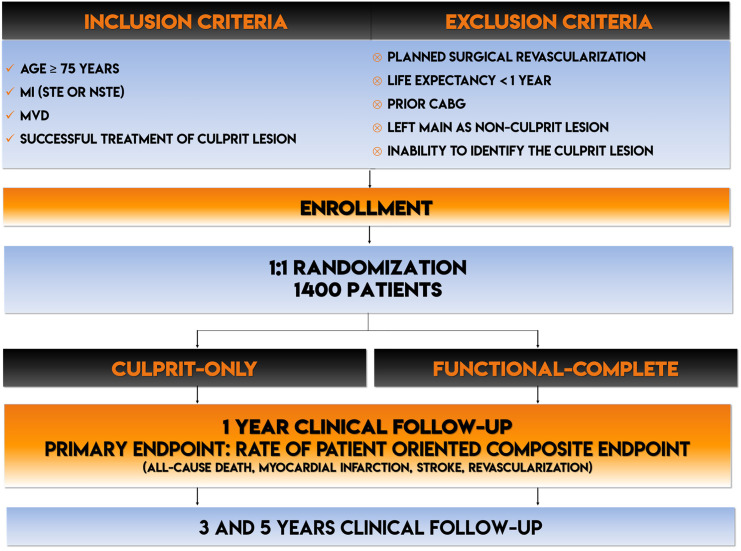
The Functional versus Culprit-only Revascularization in Elderly Patients with Myocardial Infarction and Multivessel Disease (FIRE) trial is an all-comers, prospective, randomized, international, multicenter, open-label study with blinded adjudicated evaluation of outcomes Prospective Randomized Open Blinded End-point (PROBE). The study flowchart is reported in Figure 1 . The sponsor of the study is the Italian nonprofit organization Consorzio Futuro in Ricerca (www.ciefferre.it). Institutional review boards in all participating centers have approved the protocol. Patients will be included if they meet all the following inclusion criteria: (1) age ≥ 75 years; (2) MI (STE or NSTE) with indication to invasive management; (3) MVD defined as clearly identifiable culprit lesion amenable for PCI and stenting and at least 1 lesion in a nonculprit coronary artery different from the culprit one, showing at least 2.5 mm in diameter deemed at visual estimation with a diameter stenosis percentage ranging from 50 to 99% in 2 perpendicular angiographic views and amenable to successful treatment with PCI; and (4) successful treatment of culprit lesion. The main exclusion criteria are planned surgical revascularization; noncardiovascular comorbidity reducing life expectancy to <1 year; any factor precluding 1-year follow-up; prior coronary artery bypass graft surgery; left main as nonculprit lesion; and inability to identify a clear culprit lesion (Figure 1). In the NSTEMI setting, culprit lesion identification should rely on ECG, echocardiography, and conventional angiography. In addition, invasive (intravascular ultrasound–near-infrared spectroscopy and/or optical coherence tomography) and noninvasive imaging tools (cardiac magnetic resonance and/or speckle tracking) should be used to identify the culprit lesion when the conventional assessment is not conclusive (see Supplementary Materials).
Figure 1.
Study flowchart.
YS, years.
Study procedures
Screening phase
All patients undergoing CAA because of MI must be screened for eligibility. Every month during the enrollment phase, the study team will check via a screening log the number of eligible patients not enrolled to eventually reduce the selection bias. Written informed consent must be obtained prior to randomization. The informed consent can be signed before CAA, but the patient's eligibility can be confirmed only after the evidence of MVD amenable for PCI and after the successful culprit lesion treatment. Culprit lesion PCI is defined as successful by the operator based on final flow (Thrombolysis in Myocardial Infarction flow 3); residual stenosis (<30%); and absence of clinical, angiographic, or electrocardiographic signs of complications. It also has to be assessed with 2 perpendicular angiographic views. Patients with MI and MVD receiving only culprit lesion treatment in the index procedure can be considered eligible and then randomized within 48 hours after the end of the procedure. Key baseline patient characteristics (ie, inclusion/exclusion criteria, demographics, medical history, details of cardiovascular anatomy, ECG, and laboratory test results) will be recorded on the electronic case report forms (https://trials-ice.advicepharma.com/firetrial/). All CAAs from the initial qualifying PCI as well as all functional assessments will be collected and forwarded to an angiographic core laboratory for central blinded assessment.
Randomization and treatment protocol
Randomization will be performed after CAA and culprit lesion treatment using an Internet-based system. The patient identification number and the treatment allocation will be assigned by the central randomization system. Treatment allocation will be assigned according to a computer-generated randomization list stratified by center. Randomization will also be stratified by sex and clinical presentation (STE- vs NSTE-MI). All patients who are randomized are irrevocably included in the study whether or not they are subsequently found to be eligible or actually receiving the allocated treatment. Therefore, all patients must be followed until the prespecified study end date.
General information regarding revascularization
Drug-eluting stents with biodegradable polymer with thin struts should be implanted.38 To standardize the treatment, it is strongly suggested to use Supraflex Cruz (SMT Pvt Ltd, Surat, India). Supraflex Cruz is an ultrathin (60 μm for all diameters and lengths) stent with a biodegradable polymer-eluting sirolimus. Supraflex has been demonstrated to be noninferior to the actual best in class (Xience, Abbott) in terms of device-oriented end point with a very low rate of MI (2.5%) and definite or probable stent thrombosis (0.8%). Interestingly, in the per-protocol analysis, Supraflex showed a significant 61% reduction of clinical indicated target lesion revascularization (TLR) (3.1 vs 1.2%, P = .02).40 Supraflex Cruz represents the newer version of the device with a proprietary link (LDZ-link) that should improve deliverability. A radial approach is strongly recommended. Presence of chronic total occlusion (CTO) is not an exclusion criterion per se, although CTO vessel cannot be the object of randomization. Thus, another nonculprit lesion ≥50% to randomize the patient is needed. The management of CTO in the complete revascularization group is left to the operators' discretion according to the clinical practice of their institution. All patients randomized to culprit-only revascularization must not undergo PCI any lesion except the culprit lesion already treated at the moment of the randomization. Staged procedures are considered a protocol violation. Patients who are randomized to functionally guided complete strategy will receive revascularization of the nonculprit lesions guided by functional assessment. Functional evaluation is mandatory for all stenoses with diameter stenosis percentage between 50% and 90% at visual estimation, whereas it is suggested but not mandatory for all stenoses between 91% and 99%.41 The system used to obtain functional evaluation is left to the operator's discretion. FFR, instantaneous free-wave ratio, resting full-cycle ratio, diastolic hyperemia-free ratio, diastolic pressure ratio, contrast FFR, and quantitative flow ratio are all allowed. PCI is allowed only if functional evaluation is positive according to the threshold of the chosen functional system. PCI of vessel with negative functional evaluation is considered a protocol violation. Routine stress testing and repeat angiography are not indicated in patients whose symptoms are stable. It is suggested to achieve functional complete revascularization within the index procedure, whereas it is mandatory to obtain it within the index hospitalization.
Optimal medical therapy
All patients, regardless of their randomization group, will receive OMT. Unless there is an absolute contraindication, all patients will receive standard secondary prevention according to current guidelines.42 In the presence of typical or atypical effort angina (or equivalent symptoms), β-blockers, nitrates, ranolazine, and ivabradine should be titrated before suggesting a new CAA. Patients at high bleeding risk according to the Bleeding Academic Consortium (BARC) criteria43 should be treated with a short DAPT regimen (1 month) as per prespecified substudy (www.thefiretrial.com/news FIRE-HDR substudy).
Follow-up visits
After initial hospital discharge, routine clinic follow-up will occur at 1 month ± 14 days (telephone contact or clinic visit, according to the local practice), 1 year (clinic visit), and yearly clinic visits thereafter up to 5 years. At each visit, the general status, compliance with medical therapy, and adverse events will be assessed. Low-density lipoprotein, blood pressure and glycemic targets, angina status (Seattle Angina Questionnaire), quality of life (EuroQol-5 Dimension), frailty (Rockwood Clinical Frailty Scale), and physical performance (Short Physical Performance Battery) will be assessed at the 1-, 3- and 5-year visit.
Study end points
Being a strategy trial, we identified the patient-oriented composite end point (POCE) as the primary outcome of interest (all-cause death, any MI, any stroke, any revascularization).44 The primary outcome will be assessed at 1 year. The key secondary outcome will be the composite of CV death and new MI at 1 year. MI will be defined according to the Fourth Universal Definition (see study protocol).45 All components of the primary outcome will be tested also at 3 and 5 years. A committee consisting of clinicians who are blinded to treatment allocation (Clinical Event Committee) will review and adjudicate all adverse events based on source documents. Adjudication results will be binding for the final analysis. To minimize potential bias related to open-label design, only the revascularizations fully respecting the below reported criteria will be considered. Revascularization will be considered ischemia driven and, consequently, appropriate if it is performed in the presence of hospitalization for recurrent MI; hospitalization for hemodynamic instability or refractory ischemic heart failure (defined as Killip class ≥3); intractable angina Canadian Cardiovascular Society (CCS) (class 3 or 4 symptoms) despite OMT and positive functional assessment or objective, proven, and documented evidence of ischemia in the territory of 1 or more vessels (eg, myocardial perfusion scintigraphy with ischemic territory >10% of overall left ventricular mass). Other secondary outcomes will include major bleedings according to BARC classification; EuroQol-5 Dimension quality of life; Short Physical Performance Battery, and Seattle Angina Questionnaire Frequency scale at 1, 3, and 5 years.
Statistical considerations
All statistical analyses will be performed by the Clinic and Epidemiology Research Center of the University of Ferrara. The analysis will be performed on an intention-to-treat set, defined as all intentionally randomized patients, by randomization treatment. Supportive per-protocol analyses will be performed on the primary and key secondary end points. A detailed statistical analysis plan will be completed before the end of the study and uploaded on the trial Web site. In brief, continuous variables will be tested for normal distribution with the Kolmogorov-Smirnov test and with a visual estimate of the Q-Q plot. Normally distributed variables will be presented as mean ± SD and compared by t test and 1-way analysis of variance. Otherwise, median (interquartile range), Mann-Whitney U, and Kruskal-Wallis tests will be used. Categorical variables will be summarized in terms of absolute and relative frequencies (percentages) and compared by using the χ2 test. Two-sided tests will be carried out to check the superiority of functionally driven complete revascularization. Statistical significance will be set at α = .05 level. Formal type I error control will be ensured for the primary and the key secondary end point by a sequential procedure where significance for the key secondary end point is accepted only if the primary end point is positive. Kaplan-Meier curves will be plotted to describe survival free from adverse events, and the difference between groups will be tested with the log-rank test. Cox regression models will test any confounding factor. Variables with a P value < .1 at univariate analysis will be entered into a multivariable analysis to identify the independent predictors. When appropriate, 95% CI will be calculated. In addition, we will assess the composite primary outcome POCE with the use of the Finkelstein-Schoenfeld method, which is based on the principle that each patient in the clinical trial is compared with every other patient in a pairwise manner. This method focuses primarily on all-cause mortality. The pairwise comparison proceeds in hierarchical fashion using all-cause mortality followed by frequency of MI when patients cannot be differentiated based on mortality and so on for the other end points. We will apply the Finkelstein-Schoenfeld method to the patients stratified according to clinical presentation (STE vs NSTE) and the number of diseased vessels (2 vs 3), yielding 4 stratification pools. All analyses will be performed with STATA 13 (StataCorp, College Station, TX).
Determination of sample size
Data are lacking regarding death, MI, stroke, and revascularization at 1 year in patients ≥75 years with MI and MVD treated with culprit-only revascularization. Taking into account available (Table I) and recently published data,46 , 47 we estimated a conservative 15% rate of the primary end point (POCE) at 1 year in the culprit-only strategy group. We hypothesize that the functional assessment should reduce the primary end point by at least 30% (Table II ). Therefore, the sample size required to have an 80% chance to achieve this result is of 1,358 patients, considering as significant the 5% level (computation by log-rank test). Taking into account a 2% attrition rate, the final sample size is inflated to 1,385 patients. After at least 900 patients have completed the 30-day follow-up, the assumption of the sample size calculation will be checked by estimating the Kaplan-Meier 1-year risk of having reached the primary end point. Unadjudicated data will be used for this purpose. No randomization information will be available, and all evaluations on the sample size will be performed in a blinded fashion. If the pooled event rate will be significantly lower than expected, at .01 significance level, the sample size may be increased.
Table II.
Primary end point reduction with complete revascularization in acute coronary syndrome setting
| Trial | Primary end point | HR (95% CI) |
|---|---|---|
| COMPARE-ACUTE15 | All-cause death, MI, any revascularization, and cerebrovascular events | 0.35 (0.22-0.55) |
| DANAMI-3-PRIMULTI14 | All-cause death, MI, or ischemia-driven revascularization | 0.56 (0.38-0.83) |
| COMPLETE17 | CV death, MI, or ischemia-driven revascularization | 0.51 (0.43-0.61) |
| CvLPRIT13 | All-cause death, MI, HF, and ischemia-driven revascularization | 0.45 (0.24-0.84) |
| PRAMI12 | CV death, MI, or refractory angina | 0.35 (0.21-0.58) |
HF, heart failure.
Predefined substudies
The FIRE trial program includes several prespecified substudies. The synopsis of all prespecified substudies will be uploaded on the Web site (www.thefiretrial.com) and will be freely downloadable. It is important to mention the 4 substudies that have been generated to solve specific issues and to fill the evidence gaps. The first focuses on the occurrence of adverse events in patients with high dual risk (ischemic and bleeding according to BARC high bleeding risk classification43) treated with short DAPT regimen (1 month) and sirolimus-eluting biodegradable polymer drug-eluting stent (Supraflex Cruz, SMT Pvt Ltd, Surat, India). The second and the third will investigate the performance and the additional value of the quantitative flow ratio on nonculprit lesions and of ClearStent enhanced stent visualization system (Siemens Healthcare, Munich, Germany) on stent implantation. The fourth will be the cost-effectiveness analysis in the study population.
Study organization
The FIRE trial is ongoing in sites in Italy, Spain, and Poland. Any additional site participating in the trial will be reported on the Web site in the dedicated area. The Executive Committee of the Study is composed of Simone Biscaglia (Principal Investigator), Gianluca Campo (Study Chair), Javier Escaned (Spain National Coordinator), Dariusz Dudek (Poland National Coordinator), Raul Moreno, Matteo Tebaldi, Antonio Colombo, and Emanuele Barbato. The statistical analysis will be performed by the Centre for Epidemiology and Statistics of the University of Ferrara (Elisa Maietti, Stefano Volpato) with the supervision of an external statistician (Giuseppe Biondi Zoccai).
Funding
The FIRE trial is an investigator-initiated study. The sponsor received unrestricted grants for the trial conduction from SMT, Sahajanand Medical Technologies Pvt. Ltd. India Medis, Medical Imaging. The Netherlands and Siemens Healthcare GmbH. Germany (see https://www.thefiretrial.com/supporters/ for updates). The authors are solely responsible for the design and conduct of this study, all study analyses, and the drafting and editing of the paper.
Angiographic and functional core laboratory
A central core laboratory located in the University of Ferrara will review, blinded to patient outcomes, all the angiographies from the enrolling centers, the evaluation of the culprit lesion, its successful treatment, the percentage diameter stenosis, and the functional evaluation of all the stenoses with the evaluation of the functional pitfalls. The core laboratory will also have the task of safeguarding the quality of the angiograms obtained at each participating center. Whenever relevant pitfalls in the functional evaluation are present in more than 3 cases in the same center, detailed retraining on functional evaluation will be performed. If the center will fail to perform a proper functional evaluation even after the functional retraining, any patient enrollment at the site will be stopped. The coordinator of the core laboratory (Prof Emanuele Barbato) and all the rest of the team are not directly involved in the enrollment of patients.
State of the art, timelines, and conclusions
The study was registered on December 11, 2018, with ClinicalTrials.gov identifier NCT03772743. The approval of the Ethics Committee of the coordinating center (Azienda Ospedaliera Universitaria di Ferrara, Ferrara, Italy) was obtained on January 23, 2019. The enrollment phase started on July 15, 2019. Most of the 27 active centers started the enrolment phase between October and December 2019. Within October 2020, 36 centers will be active. By July 2020, 515 patients have been enrolled. Because of the SARS-COVID 19 pandemic, the timeline for the end of the enrollment has been postponed to the second quarter of 2021 to obtain the completion of the primary end point evaluation within the second quarter of 2022. The follow-up will continue for up to 5 years.
Twitter and Web site
The idea beyond the conduction of the FIRE trial is to ensure that patients and physicians have access to transparent and interactive interfaces to be able to check the study status and the news day by day. To this end, a Web site is available (www.thefiretrial.com) with dedicated sections for physicians and patients. The Web site has also educational purposes with a dedicated section (tips) with a PowerPoint presentation of several clinical topics related to the study. In addition, the FIRE trial is on twitter (@theFIRE_trial) to amplify the study and the investigators' visibility as well as to share clinical issues related to the topic of the study with colleagues around the world.
Discussion
The prognosis of elderly patients presenting with MI and MVD is impaired because of well-known factors, including (1) high ischemic and bleeding risk profile and (2) presence of MV disease that is often left untreated, but also by (3) limited applicability of evidence-based treatment because of exclusion of elderly patients in pivotal, practice-changing RCT.
These observations have led to the design of dedicated and complementary studies with the aim to test strategies to improve the outcome of elderly MI patients. The Opportunities for Enhancing the Care of Older Patients with ST-Elevation Myocardial Infarction Presenting for Primary Percutaneous Coronary Intervention trial will be focused on older STEMI patients with MVD and will randomize them to instantaneous free-wave ratio–guided or culprit-only revascularization.48 It will include around 500 patients aged ≥60 years. The SENIOR-RITA randomizes patients aged 75 and over to invasive (CAA ± PCI) versus conservative management (NCT03052036). The FIRE trial includes patients ≥75 years with both STEMI and NSTEMI using a strategy able to reduce periprocedural complications (functional-guided complete revascularization).
Altogether, findings from these studies will be helpful to improve the management of a growing subgroup of patients admitted to hospitals.
Study limitations
We are aware that the FIRE trial has some limitations. First, the focus is on the management of elderly patients with MI and MV disease. Consequently, the randomization occurs after CAA (within 48 hours after PCI of the culprit lesion), and the study results will only be applicable to patients who underwent invasive management, thus excluding those treated conservatively (either not receiving CAA or PCI). For the same reason, the presence of selection bias cannot be ruled out, although strategies aimed at its minimization have been used (eg, monthly screening log). Second, complete revascularization has to be obtained within index hospitalization, whereas, after the recent publication of a COMPLETE subanalysis,49 a broader time frame could have also been possible. However, when the FIRE trial was designed, the available evidence at the time suggested that achieving complete revascularization within the same procedure12 , 13 , 50 or at least within index hospitalization14 could be the best strategy. Third, there are no randomized data on physiology-guided complete revascularization in NSTEMI, and this may have affected the sample size calculation. Fourth, the inclusion of STEMI and NSTEMI may complicate the interpretation of the results, although randomization is stratified according to clinical presentation. Fifth, after the publication of the COMPLETE trial,17 a primary end point including CV death and MI might have been more suitable. However, all published and ongoing studies regarding complete revascularization, including COMPLETE, had ischemia-driven revascularization in the primary end point. Sixth, the inclusion of patients with CTO is aimed at the inclusion of a real-life population, but it may add a potential confounder. Finally, randomization is performed only on the basis of age, whereas comorbidities and frailty should also be considered as important determinants of the overall patient's status46.
Disclosures
Simone Biscaglia: research grants from SMT, Medis, Eukon, Siemens; speaking or consulting fees from Bayer. Gianluca Campo: research grants from SMT, Medis, Eukon, Siemens, Astrazeneca, Guerbet, Boston Scientific, Amgen; speaking or consulting fees from Astrazeneca, Menarini, Abbott, Boston Scientific. Matteo Tebaldi: speaking or consulting fees from Abbott. Luca Fileti: speaking honoraria from Abbott, Bristol Myers Squibb. All the other authors report no disclosure.
Footnotes
Trial registration: ClinicalTrials.govNCT03772743.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ahj.2020.08.007.
Appendix. Supplementary data
Supplemental Material 1: Manuscript appendix.
Supplemental Material 2: Statistical Analysis Plan.
Supplemental Material 3: Study protocol.
References
- 1.Sidney S., Go A.S., Jaffe M.G. Association between aging of the US population and heart disease mortality from 2011 to 2017. JAMA Cardiol. 2019;4(12):1280–1286. doi: 10.1001/jamacardio.2019.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madhavan M.V., Gersh B.J., Alexander K.P. Coronary artery disease in patients >/=80 years of age. J Am Coll Cardiol. 2018;71(18):2015–2040. doi: 10.1016/j.jacc.2017.12.068. [DOI] [PubMed] [Google Scholar]
- 3.Yeh R.W., Sidney S., Chandra M. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362(23):2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 4.Garot P., Morice M.C., Tresukosol D. 2-Year outcomes of high bleeding risk patients after polymer-free drug-coated stents. J Am Coll Cardiol. 2017;69(2):162–171. doi: 10.1016/j.jacc.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Urban P., Meredith I.T., Abizaid A. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med. 2015;373(21):2038–2047. doi: 10.1056/NEJMoa1503943. [DOI] [PubMed] [Google Scholar]
- 6.Secemsky E.A., Yeh R.W., Kereiakes D.J. Mortality following cardiovascular and bleeding events occurring beyond 1 year after coronary stenting: a secondary analysis of the Dual Antiplatelet Therapy (DAPT) study. JAMA Cardiol. 2017;2(5):478–487. doi: 10.1001/jamacardio.2017.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohamed M.O., Kinnaird T., Anderson R. Combinations of bleeding and ischemic risk and their association with clinical outcomes in acute coronary syndrome. Int J Cardiol. 2019;290:7–14. doi: 10.1016/j.ijcard.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 8.de La Torre Hernandez J.M., Gomez Hospital J.A., Baz J.A. Multivessel disease in patients over 75years old with ST elevated myocardial infarction. Current management strategies and related clinical outcomes in the ESTROFA MI+75 nation-wide registry. Cardiovasc Revasc Med. 2018;19(5 Pt B):580–588. doi: 10.1016/j.carrev.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Sorajja P., Gersh B.J., Cox D.A. Impact of multivessel disease on reperfusion success and clinical outcomes in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Eur Heart J. 2007;28(14):1709–1716. doi: 10.1093/eurheartj/ehm184. [DOI] [PubMed] [Google Scholar]
- 10.Jensen L.O., Terkelsen C.J., Horvath-Puho E. Influence of multivessel disease with or without additional revascularization on mortality in patients with ST-segment elevation myocardial infarction. Am Heart J. 2015;170(1):70–78. doi: 10.1016/j.ahj.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Ibanez B., James S., Agewall S. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39(2):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 12.Wald D.S., Morris J.K., Wald N.J. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369(12):1115–1123. doi: 10.1056/NEJMoa1305520. [DOI] [PubMed] [Google Scholar]
- 13.Gershlick A.H., Khan J.N., Kelly D.J. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65(10):963–972. doi: 10.1016/j.jacc.2014.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engstrom T., Kelbaek H., Helqvist S. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386(9994):665–671. doi: 10.1016/s0140-6736(15)60648-1. [DOI] [PubMed] [Google Scholar]
- 15.Smits P.C., Abdel-Wahab M., Neumann F.J. Fractional flow reserve-guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017;376(13):1234–1244. doi: 10.1056/NEJMoa1701067. [DOI] [PubMed] [Google Scholar]
- 16.Politi L., Sgura F., Rossi R. A randomised trial of target-vessel versus multi-vessel revascularisation in ST-elevation myocardial infarction: major adverse cardiac events during long-term follow-up. Heart. 2010;96(9):662–667. doi: 10.1136/hrt.2009.177162. [DOI] [PubMed] [Google Scholar]
- 17.Mehta S.R., Wood D.A., Storey R.F. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. 2019;381(15):1411–1421. doi: 10.1056/NEJMoa1907775. [DOI] [PubMed] [Google Scholar]
- 18.Joshi F.R., Lonborg J., Sadjadieh G. The benefit of complete revascularization after primary PCI for STEMI is attenuated by increasing age: results from the DANAMI-3-PRIMULTI randomized study. Catheter Cardiovasc Interv. 2020;18 doi: 10.1002/ccd.29131. [DOI] [PubMed] [Google Scholar]
- 19.De Carlo M., Liga R. Elderly patients with non–ST-elevation acute coronary syndromes: a call for action. Heart. 2017;103(24):1932–1933. doi: 10.1136/heartjnl-2017-311694. [DOI] [PubMed] [Google Scholar]
- 20.Bauer T., Koeth O., Junger C. Effect of an invasive strategy on in-hospital outcome in elderly patients with non–ST-elevation myocardial infarction. Eur Heart J. 2007;28(23):2873–2878. doi: 10.1093/eurheartj/ehm464. [DOI] [PubMed] [Google Scholar]
- 21.Rosengren A., Wallentin L., Simoons M. Age, clinical presentation, and outcome of acute coronary syndromes in the Euroheart acute coronary syndrome survey. Eur Heart J. 2006;27(7):789–795. doi: 10.1093/eurheartj/ehi774. [DOI] [PubMed] [Google Scholar]
- 22.Reed G.W., Rossi J.E., Cannon C.P. Acute myocardial infarction. Lancet. 2017;389(10065):197–210. doi: 10.1016/S0140-6736(16)30677-8. [DOI] [PubMed] [Google Scholar]
- 23.Roffi M., Patrono C., Collet J.P. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37(3):267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 24.Alabas O.A., Jernberg T., Pujades-Rodriguez M. Statistics on mortality following acute myocardial infarction in 842 897 Europeans. Cardiovasc Res. 2020;116(1):149–157. doi: 10.1093/cvr/cvz197. [DOI] [PubMed] [Google Scholar]
- 25.Feldman L., Steg P.G., Amsallem M. Editor's choice—medically managed patients with non–ST-elevation acute myocardial infarction have heterogeneous outcomes, based on performance of angiography and extent of coronary artery disease. Eur Heart J Acute Cardiovasc Care. 2017;6(3):262–271. doi: 10.1177/2048872615626354. [DOI] [PubMed] [Google Scholar]
- 26.Rathod K.S., Koganti S., Jain A.K. Complete versus culprit-only lesion intervention in patients with acute coronary syndromes. J Am Coll Cardiol. 2018;72(17):1989–1999. doi: 10.1016/j.jacc.2018.07.089. [DOI] [PubMed] [Google Scholar]
- 27.Mariani J., Macchia A., De Abreu M. Multivessel versus single vessel angioplasty in non-ST elevation acute coronary syndromes: a systematic review and metaanalysis. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0148756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rich M.W., Chyun D.A., Skolnick A.H. Knowledge gaps in cardiovascular care of the older adult population: a scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society. J Am Coll Cardiol. 2016;67(20):2419–2440. doi: 10.1016/j.jacc.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xaplanteris P., Fournier S., Pijls N.H.J. Five-year outcomes with PCI guided by fractional flow reserve. N Engl J Med. 2018;379(3):250–259. doi: 10.1056/NEJMoa1803538. [DOI] [PubMed] [Google Scholar]
- 30.Zimmermann F.M., Omerovic E., Fournier S. Fractional flow reserve–guided percutaneous coronary intervention vs. medical therapy for patients with stable coronary lesions: meta-analysis of individual patient data. Eur Heart J. 2019;40(2):180–186. doi: 10.1093/eurheartj/ehy812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi Y., Lonborg J., Jong A. Prognostic value of the residual SYNTAX score after functionally complete revascularization in ACS. J Am Coll Cardiol. 2018;72(12):1321–1329. doi: 10.1016/j.jacc.2018.06.069. [DOI] [PubMed] [Google Scholar]
- 32.Panaich S.S., Arora S., Patel N. Comparison of in-hospital mortality, length of stay, postprocedural complications, and cost of single-vessel versus multivessel percutaneous coronary intervention in hemodynamically stable patients with ST-segment elevation myocardial infarction (from Nationwide Inpatient Sample [2006 to 2012]) Am J Cardiol. 2016;118(7):950–958. doi: 10.1016/j.amjcard.2016.06.057. [DOI] [PubMed] [Google Scholar]
- 33.Zeitouni M., Silvain J., Guedeney P. Periprocedural myocardial infarction and injury in elective coronary stenting. Eur Heart J. 2018;39(13):1100–1109. doi: 10.1093/eurheartj/ehx799. [DOI] [PubMed] [Google Scholar]
- 34.Giacoppo D., Madhavan M.V., Baber U. Impact of contrast-induced acute kidney injury after percutaneous coronary intervention on short- and long-term outcomes: pooled analysis from the HORIZONS-AMI and ACUITY trials. Circ Cardiovasc Interv. 2015;8(8) doi: 10.1161/CIRCINTERVENTIONS.114.002475. [DOI] [PubMed] [Google Scholar]
- 35.McCullough P.A., Choi J.P., Feghali G.A. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2016;68(13):1465–1473. doi: 10.1016/j.jacc.2016.05.099. [DOI] [PubMed] [Google Scholar]
- 36.Ben-Yehuda O., Chen S., Redfors B. Impact of large periprocedural myocardial infarction on mortality after percutaneous coronary intervention and coronary artery bypass grafting for left main disease: an analysis from the EXCEL trial. Eur Heart J. 2019;40(24):1930–1941. doi: 10.1093/eurheartj/ehz113. [DOI] [PubMed] [Google Scholar]
- 37.Wang T.Y., McCoy L.A., Bhatt D.L. Multivessel vs culprit-only percutaneous coronary intervention among patients 65 years or older with acute myocardial infarction. Am Heart J. 2016;172:9–18. doi: 10.1016/j.ahj.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 38.Varenne O., Cook S., Sideris G. Drug-eluting stents in elderly patients with coronary artery disease (SENIOR): a randomised single-blind trial. Lancet. 2018;391(10115):41–50. doi: 10.1016/S0140-6736(17)32713-7. [DOI] [PubMed] [Google Scholar]
- 39.Valgimigli M., Patialiakas A., Thury A. Zotarolimus-eluting versus bare-metal stents in uncertain drug-eluting stent candidates. J Am Coll Cardiol. 2015;65(8):805–815. doi: 10.1016/j.jacc.2014.11.053. [DOI] [PubMed] [Google Scholar]
- 40.Zaman A., de Winter R.J., Kogame N. Safety and efficacy of a sirolimus-eluting coronary stent with ultra-thin strut for treatment of atherosclerotic lesions (TALENT): a prospective multicentre randomised controlled trial. Lancet. 2019;393(10175):987–997. doi: 10.1016/S0140-6736(18)32467-X. [DOI] [PubMed] [Google Scholar]
- 41.Lonborg J., Engstrom T., Kelbaek H. Fractional flow reserve–guided complete revascularization improves the prognosis in patients with ST-segment-elevation myocardial infarction and severe nonculprit disease: a DANAMI 3-PRIMULTI substudy (Primary PCI in Patients With ST-Elevation Myocardial Infarction and Multivessel Disease: Treatment of Culprit Lesion Only or Complete Revascularization) Circ Cardiovasc Interv. 2017:10(4). doi: 10.1161/CIRCINTERVENTIONS.116.004460. [DOI] [PubMed] [Google Scholar]
- 42.Knuuti J., Wijns W., Saraste A. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020 Jan 14;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 43.Urban P., Mehran R., Colleran R. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the Academic Research Consortium for High Bleeding Risk. Eur Heart J. 2019;40(31):2632–2653. doi: 10.1093/eurheartj/ehz372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Garcia H.M., McFadden E.P., Farb A. Standardized end point definitions for coronary intervention trials: the Academic Research Consortium-2 consensus document. Eur Heart J. 2018;39(23):2192–2207. doi: 10.1093/eurheartj/ehy223. [DOI] [PubMed] [Google Scholar]
- 45.Thygesen K., Alpert J.S., Jaffe A.S. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72(18):2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 46.Campo G., Maietti E., Tonet E. The assessment of scales of frailty and physical performance improves prediction of major adverse cardiac events in older adults with acute coronary syndrome. J Gerontol A Biol Sci Med Sci. 2020 May 22;75(6):1113–1119. doi: 10.1093/gerona/glz123. [DOI] [PubMed] [Google Scholar]
- 47.Volz S., Petursson P., Angeras O. Prognostic impact of percutaneous coronary intervention in octogenarians with non-ST elevation myocardial infarction: A report from SWEDEHEART. Eur Heart J Acute Cardiovasc Care. 2019 Sep 13 doi: 10.1177/2048872619877287. [DOI] [PubMed] [Google Scholar]
- 48.Rymer J.A., Mandawat A., Abbott J.D. Opportunities for enhancing the care of older patients with ST-elevation myocardial infarction presenting for primary percutaneous coronary intervention: Rationale and design of the SAFE-STEMI for Seniors trial. Am Heart J. 2019;218:84–91. doi: 10.1016/j.ahj.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 49.Wood D.A., Cairns J.A., Wang J. Timing of staged nonculprit artery revascularization in patients with ST-segment elevation myocardial infarction: COMPLETE trial. J Am Coll Cardiol. 2019;74(22):2713–2723. doi: 10.1016/j.jacc.2019.09.051. [DOI] [PubMed] [Google Scholar]
- 50.Sardella G., Lucisano L., Garbo R. Single-staged compared with multi-staged PCI in multivessel NSTEMI patients: the SMILE trial. J Am Coll Cardiol. 2016;67(3):264–272. doi: 10.1016/j.jacc.2015.10.082. [DOI] [PubMed] [Google Scholar]
- 51.Dambrink J.H., Debrauwere J.P. van 't Hof AW, Ottervanger JP, Gosselink AT, Hoorntje JC, et al. Non-culprit lesions detected during primary PCI: treat invasively or follow the guidelines? EuroIntervention. 2010;5(8):968–975. [PubMed] [Google Scholar]
- 52.Hamza M., Mahmoud N., Elgendy I.Y. A Randomized trial of complete versus culprit-only revascularization during primary percutaneous coronary intervention in diabetic patients with acute ST elevation myocardial infarction and multivessel disease. J Interv Cardiol. 2016;29(3):241–247. doi: 10.1111/joic.12293. [DOI] [PubMed] [Google Scholar]
- 53.Di Mario C., Mara S., Flavio A. Single vs multivessel treatment during primary angioplasty: results of the multicentre randomised HEpacoat for cuLPrit or multivessel stenting for Acute Myocardial Infarction (HELP AMI) Study. Int J Cardiovasc Intervent. 2004;6(3–4):128–133. doi: 10.1080/14628840310030441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material 1: Manuscript appendix.
Supplemental Material 2: Statistical Analysis Plan.
Supplemental Material 3: Study protocol.