Abstract
Purpose
To investigate the clinico-radiological findings and outcomes in pregnant women with COVID-19 pneumonia compared to age-matched non-pregnant women.
Methods
A retrospective case-controlled study was conducted to review clinical and CT data of 21 pregnant and 19 age-matched non-pregnant women with COVID-19 pneumonia. Four stages of CT images were analyzed and compared based on the time interval from symptom onset: stage 1 (0–6 days), stage 2 (7–9 days), stage 3 (10–16 days), and stage 4 (>16 days). The initial and follow-up data were analyzed and compared.
Results
Compared with age-matched non-pregnant women, initial absence of fever (13/21, 62%) and normal lymphocyte count (11/21, 52%) were more frequent in pregnant group. The predominant patterns of lung lesions were pure ground-glass opacity (GGO), GGO with consolidation or reticulation, and pure consolidation in both groups. Pure consolidation on chest CT was more common at presentation in pregnant cases. Pregnant women progressed with a higher consolidation frequency compared with non-pregnant group in stage 2 (95% vs 82%). Improvement was identified in stages 3 and 4 for both groups, but consolidation was still more frequent for pregnant women in stage 4. Most patients (38/40, 95%) were grouped as mild or common type. The length of hospitalization between the two groups was similar.
Conclusion
Pregnant women with COVID-19 pneumonia did not present typical clinical features, while developing a relatively more severe disease at imaging with a slower recovery course and experiencing similar outcomes compared with the non-pregnant women.
Keywords: COVID-19, pregnancy, computed tomography, outcome
Introduction
The ongoing outbreak of coronavirus disease 2019 (COVID-19) caused by the “severe acute respiratory syndrome coronavirus 2” (SARS-CoV-2) has rapidly spread worldwide.1–3 The basic reproductive number (R0), defined as the expected number of people who will become infected by an infectious case in a susceptible population, is estimated to be 3.77.4 It is important to be vigilant about the spread of COVID-19 and implement timely effective measures to control the ongoing global outbreak. Epidemiological, clinical, and radiological characteristics of patients with COVID-19 have been preliminarily reported.5–11 According to the previous studies, initial symptoms of fever and cough, male-predominance, and lymphopenia were clinically typical and chest CT characteristics included peripherally distributed ground-glass opacity (GGO) and GGO with consolidation and/or reticulation.5 However, to the best of our knowledge, most publications involved the non-pregnant adults.
Pregnant women, as a special population with immune suppression, are reported at a higher risk to infectious diseases.12 The increased risk of complication with the 2009 pandemic H1N1 influenza virus, and the higher case-fatality rate with SARS-CoV and MERS-CoV infections was found in pregnant women compared with non-pregnant adults.13,14 To date, limited data are available about COVID-19 pneumonia during pregnancy. Previous studies have described the clinical characteristics and intrauterine vertical transmission potential in pregnant women with COVID-19 pneumonia and compared their outcomes with those of general population.15–17 The clinical and radiological characteristics as well as the outcomes of pregnant women with COVID-19 pneumonia in comparison with the gender- and age-matched population have not been reported in detail. The pregnancy complications and adverse outcomes have been reported more common among pregnant women with SARS and MERS than the age-matched non-pregnant women.14 However, the outcomes for pregnant women with COVID-19 pneumonia remained unclear.
In this case-controlled study, we aimed to investigate the clinical features, serial chest CT findings, and outcomes in pregnant women with COVID-19 pneumonia by comparing with age-matched non-pregnant women, which are crucial for profiling the disease in the special populations seeking the early detection, accurate diagnosis, and timely obstetrical management.
Materials and Methods
Patients
Clinical and CT data of 33 pregnant women and 27 non-pregnant women with laboratory-confirmed COVID-19 pneumonia between 20 and 49 years old from January 23 to March 4, 2020 in the two enrolled centers were retrospectively reviewed. The exclusion criteria were as follows: (a) lack of complete clinical records (n= 1); (b) lack of follow-up data (n= 16); (c) simultaneous infection with any other common pathogen such as mycoplasma pneumonia, chlamydia pneumonia, respiratory syncytial virus, adenovirus, coxsackie virus B, influenza A, influenza B, or human parainfluenza virus (n= 1, co-infection with influenza A); (d) insufficient image quality due to the severe artifacts affecting the image assessment (n= 2). Finally, 21 pregnant and 19 age-matched non-pregnant women were recruited in this study. One was from Xinhua Hospital affiliated to Shanghai Jiao Tong University School of Medicine and the others from Maternal and Child Health Hospital of Hubei Province. Reverse-transcription polymerase chain reaction (RT-PCR) tests were performed using pharyngeal swab samples twice for each patient at 1 to 6 days after symptom onset by the local Center for Disease Prevention and Control following the WHO guideline.18 Clinical characteristics, CT features, and outcomes were compared between the two groups.
According to the Chinese Management Guideline for COVID-19 (version 6.0),19 the clinical severity of COVID-19 was grouped into four types: 1) Mild type, slight clinical symptoms but no imaging presentations of pneumonia; 2) Common type, fever, respiratory symptoms and imaging presentations of pneumonia; 3) Severe type, any of the following: respiratory distress with respiratory rate ≥30 breaths per minute, oxygen saturation ≤93% in a resting state or arteria oxygen tension (PaO2)/inspiratory oxygen fraction (FiO2) ≤300 mmHg (1mmHg=0.133kPa); 4) Critically severe type, any of the following: respiratory failure needing mechanical ventilation, shock, or combination with other organ failure needing intensive care unit (ICU) monitoring and treatment.
CT Image Acquisition
All patients underwent non-enhanced chest CT scans in the supine position during end-inspiration, using one of the 64-section multi-detector CT scanners (uCT780, United imaging, Shanghai, China, or Optima 660, GE Medical System, Milwaukee, WI, USA). The CT scan covered from thoracic entrance to the level of posterior costophrenic angle. The parameters were as follows: tube voltage 120 kV, automatic tube current (100–380) mA, thickness (5–10) mm, slice interval 5 mm, rotation speed 0.5 s or 0.6 s, and helical pitch 1.0875:1 or 1.375:1. The noise index (NI) and dose-length product (DLP) were 15 and 50–150 mGy·cm for pregnant women, while 7.99 and 250–400 mGy·cm for non-pregnant women. Thyroid, abdomen, and pelvis were protected by lead sheath during CT examinations. Lung window images at 0.625 to 1 mm thickness were reconstructed. Iterative reconstruction technique was implemented. The informed consents for CT examination were obtained from all patients.
Image Interpretation
Image analysis was performed by two experienced radiologists in consensus (HL and FL, with 8 and 15 years of experience in thoracic imaging, respectively). The disputes between the radiologists were resolved by consulting another experienced radiologist (DW or WL, with more than 25 years of experience in thoracic imaging, respectively). The radiologists were blinded to the pregnancy status of patients.
CT features consisted of lesion number, distribution, and density. The lesion was counted as the involved lung segments if a lesion extends across multiple lung segments.7 Distribution included unilateral or bilateral lungs, and peripheral or central locations. Density included pure GGO, GGO with consolidation, GGO with reticulation (intralobular/interlobular septal thickening), pure consolidation, air bronchogram, and cavitation. Lymphadenopathy within the mediastinum and pleural effusion were also analyzed. Peripheral distribution was defined as the outer one-third of the lung and lymphadenopathy as the size of lymph node more than 10 mm in short-axis diameter.
A CT scoring method was used to quantify the extent of lesions. Each of the 5 lung lobe involvement was scored from 0 to 5 as follows: 0 as none involvement; 1 as <5% involvement; 2 as 5%-25% involvement; 3 as 26%-49% involvement; 4 as 50%-75% involvement; and 5 as >75% involvement.20 The total CT score was obtained by summing the scores of the five lobes ranging from 0 to 25.
Follow-Up CT
According to the previous reported time intervals of disease progression stages,8,21 the baseline images (stage 1, 0–6 days) and 3 serial follow-up images including stage 2 (7–9 days), stage 3 (10–16 days), and stage 4 (>16 days) were comparatively analyzed based on the time intervals from symptom onset. The imaging parameters were the same as the initial baseline CT imaging. The images were reviewed by the same radiologists in consensus (HL and FL). The CT scores of both lungs and presence of lesions were assessed and compared with previous CT findings to evaluate the recovery course.
Results
Clinical Characteristics
The clinical characteristics and outcomes of 40 female patients are demonstrated in Table 1. The pregnant and case-controlled groups were matched for gender, age, and underlying illness. The mean age of the pregnant and non-pregnant groups was (31±1) years and (32±1) years. There were no underlying chronic diseases among all patients, but two pregnant women had gestational diabetes and three had gestational hypertension with two accompanied by mild pre-eclampsia or abnormal liver and cardiac function.
Table 1.
Clinical Characteristics and Outcomes of the Pregnant and Non-Pregnant Women with COVID-19 Pneumonia
| Characteristics | Pregnant Group (n=21) | Non-Pregnant Group (n=19) |
|---|---|---|
| Age, years | 31(1) | 32 (1) |
| Exposure to confirmed cases | 8 (38%) | 6 (32%) |
| Initial symptoms | ||
| Fever | 8 (38%) | 14 (74%) |
| Cough | 6 (29%) | 8 (42%) |
| Short of breath | 1 (5%) | 1 (5%) |
| Fatigue | 8 (38%) | 3 (16%) |
| Loss of appetite | 2 (10%) | 0 (0%) |
| Laboratory test | ||
| Leukocytosis | 11 (52%) | 0 (0%) |
| Leukopenia | 0 (0%) | 8 (42%) |
| Elevated neutrophil ratio | 18 (86%) | 3 (16%) |
| Lymphopenia | 10 (48%) | 15 (79%) |
| Decreased lymphocyte ratio | 16 (76%) | 15 (79%) |
| Elevated C-reactive protein | 15 (71%) | 10 (53%) |
| ICU admission | 1 (5%) | 0 (0%) |
| Mechanical ventilation | 1 (5%) | 1 (5%) |
| Discharge patients | 19 (90%) | 16 (84%) |
| Length of hospitalization (days) | 20 (2) | 17 (2) |
Note: Data are shown as mean (standard deviation) or number (percentage).
Abbreviation: ICU, intensive care unit.
The gestational age on admission was 16 weeks for one pregnant woman, and the others were in their third trimester at presentation ranging from 24 weeks to 40 weeks plus 4 days (median gestational age, 37 weeks). The common symptoms were fever, cough, and fatigue in both groups. Compared with non-pregnant women, initially normal body temperature, leukocytosis, elevated neutrophil ratio, and normal lymphocyte count were more frequent in the pregnant group. None had leukopenia. All pregnant women lived in Wuhan and eight of them were associated with familial clusters.
Totally 16 pregnant women underwent delivery, ten of whom delivered ten full-term infants. Among them, five underwent cesarean sections and the other five were vaginal delivery. Seven neonates (five singletons and two twins) born to the remaining six mothers were delivered premature between 34–36 weeks of gestation. The 1-min and 5-min Apgar scores for all the 17 neonates were 8–9 and 9–10. The causes of premature birth were as follows: intrauterine distress (n=1, 6%), premature rupture of membrane (n=3, 19%), and premature labour at 35 or 36 weeks of gestation (n=2, 13%). One pregnant woman was admitted to the ICU after cesarean section. In the non-pregnant group, one patient used non-invasive mechanical ventilation.
Six pregnant women with symptoms occurring after delivery received antibiotics and antivirals treatment. Other pregnant women with prepartum symptoms onset received only antibiotics before delivery and then combination of antibiotics and antivirals therapy after delivery. For the non-pregnant women, all the patients received antiviral treatment and ten received combination antibiotics treatment. Antibiotics included cephalosporins, quinolones, and macrolides, and the antivirals included oseltamivir and ganciclovir. By the end of this study, two pregnant and three non-pregnant women were still SARS-CoV-2 positive and hospitalized for treatment. For the other patients, the length of hospitalization between the pregnant and non-pregnant women was similar.
Serial Chest CT
Initial CT Findings
Initial CT features of the 40 patients are summarized in Table 2. The median intervals of the initial CT scans from symptom onset were two days (inter-quartile range [IQR] 1–4, range 1–6) and two days (IQR 1–4, range 1–5) in the pregnant and non-pregnant groups. Seven pregnant women underwent initial CT scans after delivery and the others prior to delivery. There were 247 lesions detected in 60 involved lobes and 136 segments in the pregnant cases, while 92, 42, and 70 in the non-pregnant group. Peripheral and lower lobe distributions were predominant in both pregnant (76%, 51%) and non-pregnant groups (78%, 66%). Bilateral distribution (57%) and five lobes involvement (33%) were relatively common in the pregnant cases, whereas unilateral distribution (58%) and one lobe involvement (37%) were more frequent in the non-pregnant group.
Table 2.
Initial Chest CT Imaging Characteristics of the Pregnant and Non-Pregnant Women with COVID-19 Pneumonia
| Characteristics | Pregnant Group (n=21) | Non-Pregnant Group (n=19) |
|---|---|---|
| Location | ||
| None | 2 (10%) | 1 (5%) |
| Unilateral lung | 7 (33%) | 11 (58%) |
| Bilateral lungs | 12 (57%) | 7 (37%) |
| Lung lobe involvement | ||
| None | 2 (10%) | 1 (5%) |
| One lobe | 5 (24%) | 7 (37%) |
| Two lobes | 4 (19%) | 5 (26%) |
| Three lobes | 0 (0%) | 2 (11%) |
| Four lobes | 3 (14%) | 1 (5%) |
| Five lobes | 7 (33%) | 3 (16%) |
| Predominant distribution* | ||
| Peripheral | 188 (76%) | 72 (78%) |
| Central | 59 (24%) | 20 (22%) |
| Main presence of lesions* | ||
| Pure GGO | 141 (57%) | 57 (62%) |
| GGO with consolidation | 54 (22%) | 31 (34%) |
| GGO with reticulation | 40 (16%) | 41 (45%) |
| Pure consolidation | 52 (21%) | 4 (4%) |
| Air bronchogram | 15 (6%) | 11 (12%) |
| Other findings | ||
| Pleural effusion | 7 (33%) | 0 (0%) |
| Lymphadenopathy | 0 (0%) | 0 (0%) |
Notes: Data are shown as number (percentage). *Differences of Chest CT regarding lesion number between the pregnant women and age-matched non-pregnant women with COVID-19 pneumonia. The others were compared for patients.
The most common lesions were pure GGO, GGO with consolidation or reticulation, and pure consolidation in both groups. GGO lesions were the predominant pattern; however, pure consolidation had a higher incidence in the pregnant cases (Figure 1), whereas GGO with consolidation and reticulation were more frequent in the non-pregnant group (Figure 2). Initial normal CT was found in two pregnant and one non-pregnant woman. Minimal pleural effusion was present in seven pregnant women after delivery. Pulmonary edema was not detected in those two patients with mild pre-eclampsia. No cavitation and mediastinal lymphadenopathy were identified. The mean CT scores for the whole cohort, pregnant, and non-pregnant women were 4.5±3.6, 5.2±0.8, and 3.7±0.7, respectively. The CT score for pregnant group seemed to be higher compared with that for non-pregnant women.
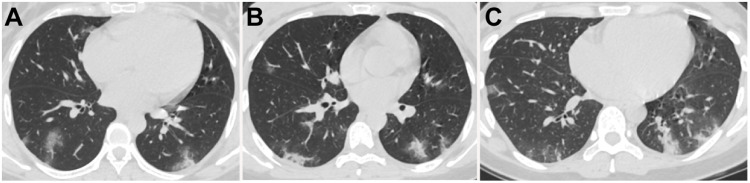
Figure 1.
Chest CT images of three pregnant women with COVID-19 pneumonia. (A) 28-year-old female with 34 weeks plus 5 days of gestation, presenting fever for 4 days. CT demonstrated scattered pure consolidation in both lungs with predominantly peripheral distribution. (B) 30-year-old female with 31 weeks of gestation, presenting fever for 2 days. Scattered pure consolidation lesions in both lungs with peripheral distribution were detected. (C) 28-year-old female with 24 weeks pregnancy, presenting fever for 2 days. Scattered ground-glass opacity (GGO) with consolidation and pure consolidation lesions in both lungs with peripheral distribution were demonstrated.
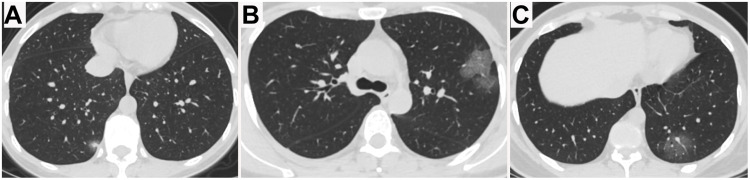
Figure 2.
Chest CT images of two non-pregnant women with COVID-19 pneumonia. (A) 36-year-old female presenting fever and cough for 4 days. Only single focal ground-glass opacity (GGO) with consolidation was detected in the right lower lobe peripherally at presentation. (B, C) 34-year-old female presenting fever for 5 days. Multifocal pure GGOs with intralesional vessel enlargement were identified in the left upper and lower lobes with peripheral distribution.
Follow-Up CT Findings
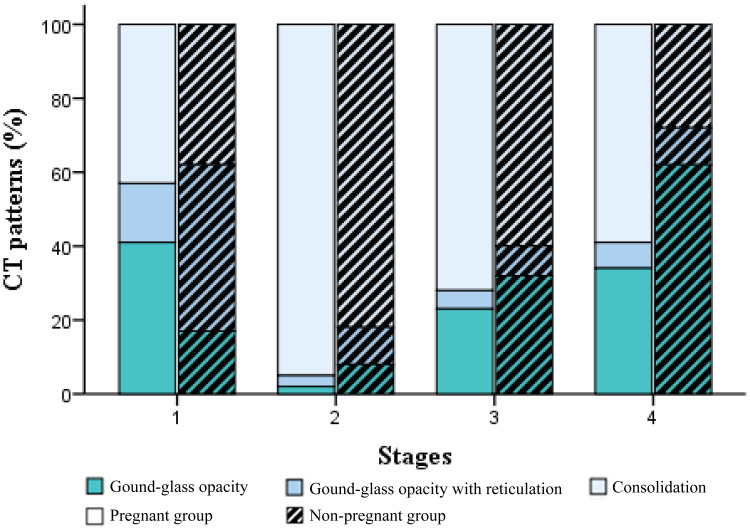
Besides the baseline chest CT study in all 40 women (stage 1), serial follow-up CT studies were performed in 27, 22, and 28 patients for stages 2–4, respectively. For the pregnant women after delivery, follow-up CT was usually performed on approximately 3,7,14, or 21 days after treatment according to the disease severity. For the pregnant women prior to delivery, follow-up CT was performed at approximately 14 or 21 days after treatment. The incidences of GGO, GGO with reticulation, and consolidation (including mixed and pure consolidation) of the four stages are demonstrated in Figure 3. Incidence of consolidation or reticulation in the initial pure GGO lesions, more pure consolidation lesions, or larger extent of lesions indicated more severity during the follow-up. In stage 1, GGO was predominant in both groups. The CT scores were 5.2±0.8 and 3.7±0.7 for pregnant and non-pregnant groups, respectively. In stage 2, 12/13 (92%) pregnant and 12/14 (86%) non-pregnant women presented progression. More GGOs, mixed GGOs with consolidation, and more pure consolidations were identified during follow-up (Figures 4 and 5). CT scores for the pregnant and non-pregnant women were 5.6±4.2 and 4.2±3.5. However, the consolidation increased to 95% and 82% from 27% and 34% in stage 1 for the pregnant and non-pregnant groups. In stage 3, 8/11 (73%) pregnant and 9/11 (82%) non-pregnant women harvested improvement. CT scores for the pregnant and non-pregnant women were 5.0±2.9 and 3.5±3.0, and incidence of consolidation was 72% and 60%. Continuous improvement was observed in 13/13 pregnant and 15/15 non-pregnant women in stage 4, in which CT scores were 3.4±3.5 and 2.6±2.8 and the consolidation frequency decreased to 59% and 28% in the above groups. Notably, consolidation was still more common in the pregnant group in the final stage.
Figure 3.
CT patterns of different stages based on the time intervals from symptom onset in the pregnant and age-matched non-pregnant groups. Stacked bars represented the proportion of lesions including ground-glass opacity (GGO), GGO with reticulation, and consolidation (mixed and pure consolidation). Four stages based on the time intervals from symptom onset were depicted: stage 1(0–6 days), stage 2 (7–9 days), stage 3 (10–16 days), and stage 4 (>16 days).
Figure 4.
Chest CT images of a 38-year-old non-pregnant female with COVID-19 pneumonia, presenting cough for 1 day. (A) Day 1 after symptom onset, ground-glass opacity (GGO) with consolidation, intralobular and interlobular septal thickening, and air bronchogram in the peripheral region of the right lower lobe were detected. (B) Day 7, the extent and density of the lesions decreased, showing GGO and irregular linear opacities. (C) Day 14, the lesions were dissipated into GGO. (D) Day 20, further resolution of the lesions.
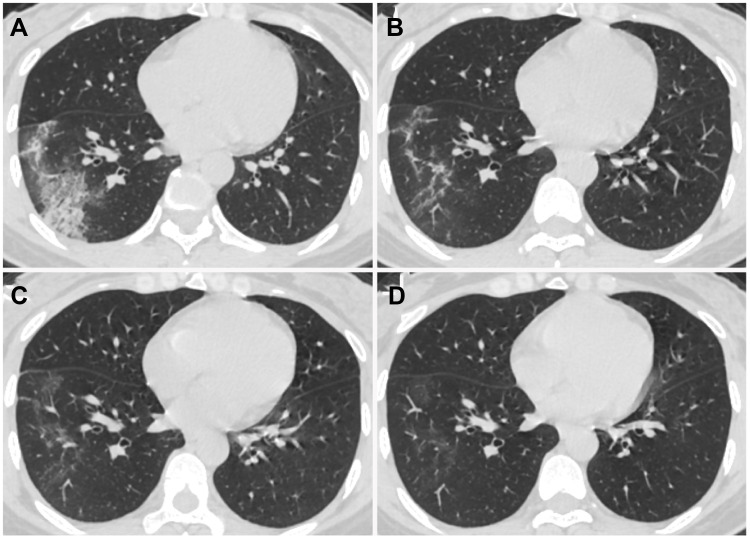
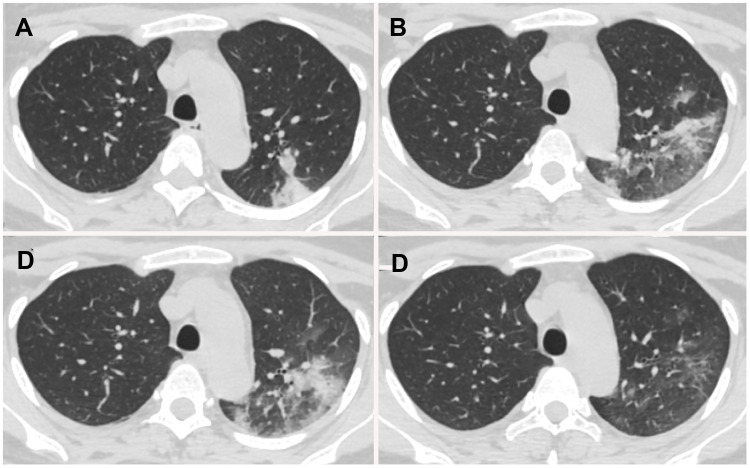
Figure 5.
Chest CT images of a 29-year-old perinatal female with COVID-19 pneumonia, presenting post-partum fever for 1 day. (A) Day 1 after symptom onset, pure consolidation was located peripherally in the left upper lobe. (B) Day 7, the lesion progressed with increased extent and heterogeneous density. (C) Day 11, the lesion further progressed with increased consolidation component. (D) Day 18, lesions were dissipated into ground-glass opacity and few irregular linear opacities.
Discussion
To the best of our knowledge, this case-controlled study is the first case series to compare the clinico-radiological features and outcomes between pregnant and age-matched non-pregnant women. Our study found that initial absence of fever and normal lymphocyte count were more frequent for pregnant women compared with the controlled cases. More severe COVID-19 pneumonia was initially identified at imaging in pregnant women with more frequent pure consolidation. Regarding the recovery, pregnant women experienced comparatively more severe progression and a slower recovery course while there were no significant adverse outcomes for the pregnant women.
For pregnant women, specific physiological and anatomical changes during pregnancy involved almost all organ systems, such as increased heart rate and oxygen consumption, decreased functional residual capacity of lungs, immunologic adaptations, etc. As demonstrated in our study, although 6 pregnant women presented post-partum fever, the initial fever was detected in only 38% of patients compared with 74% in the non-pregnant group, which could be contributed to the slow or weak response to the virus in the immunosuppressive condition during pregnancy. Leukocytosis and elevated neutrophil ratio were presented in 52% and 86% of pregnant women, while the incidence of lymphopenia was lower in the pregnant group, which was not characteristic in patients with COVID-19 pneumonia. These discrepancies could be ascribed to physiological phenomena in pregnancy.22 During this ongoing outbreak, fever is the principal surveillance index for COVID-19, and laboratory results including leukopenia or normal blood white cell count as well as lymphopenia were listed as the typical clinical findings.5 Therefore, the physiological adaptions to pregnancy make it challenging to early identify the COVID-19 in pregnant women if without any additional evidence. Hence, early diagnosis, isolation, and treatment are crucial for the pregnant women and neonates. CT plays an important role in the surveillance for COVID-19 with a higher sensitivity and time efficiency superior to RT-PCR,23 which can be regarded as the modality of choice for pregnant women in the third trimester with high suspicion of COVID-19, especially in the epidemic area.
In our cohort, 14 of 21 pregnant women were performed chest CT imaging prior to delivery. Low dose technique was implemented with DLP ranging from 50 to 150 mGy·cm for diagnostic imaging, which was safe for the fetuses.24 The mean age of enrolled patients was younger (32 years, rang 23–41) and the mean CT score was lower (4.5) than the older patients (51 years, 9.9).25 Most patients (38/40, 95%) were grouped as the mild or common types except two patients developed severe pneumonia using mechanical ventilation in our cases. Our results were consistent with previous studies reporting that younger patients experienced less severe COVID-19 pneumonia compared with the old-age people (>50 years) with comorbidities.10,11,26 When compared between the pregnant and aged-matched non-pregnant groups, the patterns of lesions were similar, including pure GGOs, GGOs with consolidation or reticulation, and pure consolidations. The presence of lesions was also similar to the general population as our previous study reported.27 These imaging features were in concordance with the pathological findings of COVID-19,28 manifesting that diffuse type II alveolar epithelial proliferation, intra-alveolar serous exudates, hyaline membrane formation, and alveolar septum edema, along with intraluminal bronchial mucous plug, mononuclear inflammatory infiltrates (dominated by lymphocytes), and fibrosis in pulmonary interstitium. However, the lung involvement was a bit more severe for pregnant women with a higher incidence of consolidation compared to the age-matched group. Some reasons could be responsible for this phenomenon. For one thing, the immunosuppressive status of pregnant women and the physiological adaptive changes in pregnancy such as dilatation of respiratory airways, and hyperemia and edema of the mucosal surfaces mediated by elevated progesterone, and decreased functional residual capacity increased the risk to COVID-19 infection. For the other, the asymptomatic status and laboratory tests could delay the recognition and diagnosis of this disease.
As for the recovery course, the progression stage of 7–9 days from symptom onset was similar in both groups, which could be reflected by the elevated CT scores and increased proportion of consolidation. The improvement was observed during stage 3 (10–16 days), which was depicted by the decreased CT scores and frequency of consolidation in both groups. The pregnant women experienced more severe progression and a slower recovery course. The incidence of consolidation was higher for the pregnant group in stage 2 after antiviral treatment, and the frequency of consolidation was also higher in stage 4 (>16 days) after continuous treatment. We speculated that the immunosuppressive status may affect the recovery course with superimposition of compromised medications for treatment, which were supposed to avoid impairment on fetuses in pregnancy. Fortunately, there was no evidence that the clinical outcomes were obviously worse for pregnant than the age-matched non-pregnant women.
In our study, CT was proved as a useful tool in diagnosing and monitoring COVID-19 pneumonia in pregnant women under the extraordinary circumstances during COVID-19 pandemic for better maternal and fetal outcomes. Although the low dose technique and a relatively long time interval for follow-up CT were implemented, and the total radiation dose was less than 50 mGy, which was reported to be not associated with the possible dose-dependent adverse outcomes such as fetal demise, growth restriction, organ malformations, and cognitive deficits.24 Anxiety could be raised among certain pregnant women due to the increased radiation dose during serial follow-up CT scans. Recently, lung ultrasound, a radiation-free tool, was found to be useful in screening and diagnosing COVID-19 in pregnant women, especially for the asymptomatic patients on presentation accounting for approximately one-third of all pregnant women in the United States.29–31 Effective screening of COVID-19 in asymptomatic pregnant women could avoid the delayed diagnosis and management. However, lung ultrasound plays a limited role in detecting lesions located in the central or inner zone areas. Furthermore, the treatment assessment of COVID-19 pneumonia could be affected by the lack of standardized patient’s positions and different experiences of operators. Hence, an applicable screening, diagnostic, and monitoring process of COVID-19 pneumonia can be developed by choosing the best modality of lung ultrasound or CT examination for better maternal and fetal outcomes.
When compared to the reported outcomes of pregnant women with SARS or MERS infections, the prognosis of patients with COVID-19 in our study was better. Wong et al32 reported that 25% of pregnant women with SARS died, and 50% were admitted to ICU. Besides, 57% of pregnancy with first-trimester infections and 80% with third-trimester infections ended in spontaneous abortion and preterm delivery. Mechanical ventilation was applied three times more than that in non-pregnant women with SARS.33 For pregnant women with MERS, 54% of patients were admitted to ICU, while 38% required ventilator support, and 23% died after delivery.14 In our study, one case in each group developed severe COVID-19 pneumonia requiring mechanical ventilation, which was mostly dissipated at follow-up chest CT after 21 and 18 days from admission. No patient died in either group. Preterm delivery occurred in six pregnant women (38%), and intrauterine distress occurred in one fetus (6%), whereas the premature rupture of membranes in three cases with suspected infection (19%), which were less common compared with previous studies.15,16
Our study had several limitations. Firstly, the sample size was relatively small, larger population from more centers should be warranted to validate our preliminary results. Secondly, we tried our best to match the time intervals of follow-up CT from symptom onset in two groups. However, the initial prepartum absence of fever occurred in some pregnant women and the complicated status of pregnant women made it difficult to match the follow-up time for each patient. Thirdly, the treatment strategies were different for pregnant women, which could affect the evaluation of clinical outcomes. However, although the pregnant women did not receive antiviral treatment before delivery, all the pregnant women achieved good outcomes similar to the non-pregnant women. Finally, as for the outcomes, the follow-up term in our study was relatively short. The long-term follow-up for the effects of COVID-19 should be further investigated.
Conclusions
Our preliminary study revealed that the clinical symptoms and laboratory tests of COVID-19 in pregnant women were not characteristic compared with the age-matched non-pregnant women, which was challenging in a battle for screening the COVID-19 in pregnancy. Lung involvement was a bit more severe in the pregnant women at presentation and the patients were supposed to take a relatively longer recovery course, even though the prognosis was not poorer than that of the non-pregnant women. Therefore, early detection, diagnosis, and timely obstetrical management are crucial for better maternal and fetal outcomes.
Funding Statement
This work was supported by Joint fund project of Hubei Health Commission [No.WJ2019H294], National Key Research and Development Program of China [No.2017YFC0109003], National Natural Science Foundation of China [No. 81901695] and Shanghai Sailing Program [No.19YF1433100]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
COVID-19, Coronavirus disease 2019; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; GGO, Ground-glass opacity; RT-PCR, Reverse-transcription polymerase chain reaction; IQR, Inter-quartile range.
Data Sharing Statement
All data generated or analyzed of this study are included in this published article. Raw and processed data during the current study are available from the corresponding authors upon reasonable request.
Ethics Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Maternal and Child Health Hospital of Hubei Province (12/3/2020, 2020-IEC-LW014) and Xinhua Hospital affiliated to Shanghai Jiao Tong University School of Medicine (21/2/2020, XHEC-C-2020-072). We performed a retrospective analysis of patient clinical information and did not conduct experiments on patient specimens. All patient data were maintained with confidentiality. This retrospective study evaluated de-identified data and involved no potential risk to patients. Therefore, the Institutional Ethics Committee waived the need for written informed consent provided by participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors declare no potential conflict of interest relevant to this article.
References
- 1.World Health Organization. WHO Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020. 2020. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020. Accessed February11, 2020.
- 2.World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19-11 March 2020; 2020. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19–11-march-2020., Accessed March11, 2020.
- 3.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y, Lu Q, Liu M, et al. Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China. medRxiv. 2020;2020(02):10.20021675. [Google Scholar]
- 5.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv. 2020;2020(02):06.20020974. [Google Scholar]
- 6.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song F, Shi N, Shan F, et al. Emerging coronavirus 2019-nCoV pneumonia. Radiology. 2020;295(1):210–217. doi: 10.1148/radiol.2020200274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang W, Cao Q, Qin L, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80(4):388–393. doi: 10.1016/j.jinf.2020.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2020. doi: 10.1093/cid/ciaa270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221(1):80–87. doi: 10.1111/j.1749-6632.2010.05938.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz DA, Graham AL. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12(2):2. doi: 10.3390/v12020194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasmussen SA, Smulian JC, Lednicky JA, Wen TS, Jamieson DJ. Coronavirus Disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. 2020. doi: 10.1016/j.ajog.2020.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9(1):51–60. doi: 10.21037/tp.2020.02.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu D, Li L, Wu X, et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. 2020:1–6. doi: 10.2214/AJR.20.23072 [DOI] [PubMed] [Google Scholar]
- 18.FDA. Fact sheet for healthcare providers: CDC – 2019-nCoV real-time RT-PCR diagnostic panel. Available from: https://www.fda.gov/media/134920/download. Accessed February 6, 2020.
- 19.National Health Commission of the People’s Republic of China. Chinese management guideline for COVID-19 (version 6.0).2020. Available from: http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b817.pdf. Accessed February19, 2020.
- 20.Chang YC, Yu CJ, Chang SC, et al. Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: evaluation with thin-section CT. Radiology. 2005;236(3):1067–1075. doi: 10.1148/radiol.2363040958 [DOI] [PubMed] [Google Scholar]
- 21.Pan F, Ye T, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020:200370. doi: 10.1148/radiol.2020200370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kazma JM, van den Anker J, Allegaert K, Dallmann A, Ahmadzia H. Anatomical and physiological alterations of pregnancy. J Pharmacokinet Pharmacodyn. 2020. doi: 10.1007/s10928-020-09677-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020:200642. doi: 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toma P, Bartoloni A, Salerno S, et al. Protecting sensitive patient groups from imaging using ionizing radiation: effects during pregnancy, in fetal life and childhood. Radiol Med. 2019;124(8):736–744. doi: 10.1007/s11547-019-01034-8 [DOI] [PubMed] [Google Scholar]
- 25.Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology. 2020;295(1):202–207. doi: 10.1148/radiol.2020200230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020;55(6):327–331. doi: 10.1097/RLI.0000000000000672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Liu F, Li J, Zhang T, Wang D, Lan W. Clinical and CT imaging features of the COVID-19 pneumonia: focus on pregnant women and children. J Infect. 2020;80(5):e7–e13. doi: 10.1016/j.jinf.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moro F, Buonsenso D, Moruzzi MC, et al. How to perform lung ultrasound in pregnant women with suspected COVID-19. Ultrasound Obstet Gynecol. 2020;55(5):593–598. doi: 10.1002/uog.22028 [DOI] [PubMed] [Google Scholar]
- 30.Yassa M, Birol P, Mutlu AM, et al. Lung ultrasound can influence the clinical treatment of pregnant women with COVID-19. J Ultrasound Med. 2020. doi: 10.1002/jum.15367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalafat E, Yaprak E, Cinar G, et al. Lung ultrasound and computed tomographic findings in pregnant woman with COVID-19. Ultrasound Obstet Gynecol. 2020;55(6):835–837. doi: 10.1002/uog.22034 [DOI] [PubMed] [Google Scholar]
- 32.Wong SF, Chow KM, Leung TN, et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191(1):292–297. doi: 10.1016/j.ajog.2003.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lam CM, Wong SF, Leung TN, et al. A case-controlled study comparing clinical course and outcomes of pregnant and non-pregnant women with severe acute respiratory syndrome. BJOG. 2004;111(8):771–774. doi: 10.1111/j.1471-0528.2004.00199.x [DOI] [PMC free article] [PubMed] [Google Scholar]