Abstract
Purpose:
To evaluate the association between baseline psychosocial milieu and subsequent glaucoma medication adherence among participants in the Support, Educate, Empower (SEE) personalized glaucoma coaching program pilot study.
Design:
Prospective cohort study.
Participants:
University of Michigan glaucoma patients ≥ age 40, taking ≥ 1 glaucoma medication, who self-reported poor adherence.
Methods:
Participants completed a baseline survey that assessed: 1) Demographics; 2) Social network; 3) Perceived Stress; 4) Consideration of future consequences; 5) Glaucoma-related distress and 6) Social support. Medication adherence was then monitored electronically (AdhereTech, New York, NY) for 3 months and the percentage of prescribed doses taken was calculated. The relationship between baseline factors and medication adherence was assessed using univariate and multivariate analysis.
Main Outcome Measures:
Median percent adherence over three months.
Results:
Of the 95 study participants, 63% had graduated from college, 55% were white, 35% were African-American, and 97% had insurance. Median adherence over three months was 74% ± 21% (± standard deviation, SD). Higher income and more education were significantly associated with better adherence (p<0.0001, p = 0.03). Glaucoma related distress (mean score 5.6, SD = 3.0) was inversely associated with medication adherence on univariate (p<0.0001) and multivariate analysis (p=0.0002). Every one-point increase in glaucoma related distress score predicted a 2.4 percentage-point decrease in medication adherence.
Conclusions:
Lower income, lower educational attainment and a higher level of glaucoma-related distress all predicted lower adherence to glaucoma medications. Additional glaucoma self-management support resources should be directed towards patients with such risk factors for poor adherence.
Poor adherence to therapeutic regimens among adults with chronic conditions is responsible for substantial morbidity, mortality, and increased health care costs in the United States (US).1 Interventions that improve adherence have improved clinical outcomes such as blood pressure,2 blood glucose,3 and blood lipids.4,5 Glaucoma remains the second leading cause of blindness in the US despite the availability of effective treatments.6,7 As with other chronic conditions, rates of poor adherence to glaucoma medications are high, and patients with worse adherence have more severe glaucoma-related vision loss.8
The Ecological Model of Health posits that health behaviors, such as medication taking, are determined by social factors, such as public policies and social support systems, and by individual-level factors such as knowledge, skills, attitudes, and self-efficacy. In focus groups, glaucoma patients have identified both social barriers to care, such as a lack of emotional support and financial difficulties, and individual barriers to care, such as poor insight into the natural history of glaucoma and low self-efficacy about self-administering eye drops.9-12 More complex dosing regimens have been found to have a statistically significant association with poorer adherence.13,14 Being of minority race, low-income, low-educational attainment, or not being married have all been found to be significantly associated with worse glaucoma medication adherence.15,16 The literature on psychosocial determinants of glaucoma medication adherence is less robust but does suggest that patients with low motivation, low intention, and poor self-efficacy also have a higher risk of poor medication adherence 17-19 To date, however, the relationships between glaucoma medication adherence and individual level factors such as perceived stress, future orientation, and disease related distress and social factors such as support network have yet to be assessed.
Accordingly, the aim of the current study is to understand the relationship between baseline individual level factors including perceived stress, future orientation, and disease related distress and social factors such as demographics and social support and glaucoma medication adherence among participants in the Support, Educate, Empower (SEE) personalized glaucoma coaching program pilot study. Specifically, we will assess the relationships between electronically monitored glaucoma medication adherence and income, race, education, perceived stress, disease-related distress, insight into future consequences, social network and social support among participants in the SEE program pilot study. As the purpose of the SEE program is to improve glaucoma medication adherence, only participants with poor self-reported medication adherence were included. Understanding the baseline psychosocial correlates of medication adherence among program participants will better inform how to best identify which glaucoma patients would benefit from additional support.
Methods
This study examined the baseline demographics and psychosocial characteristics collected from a cohort enrolled in the SEE Program pilot study (NCT03159247), a prospective study examining the initial impact and feasibility of a personalized glaucoma coaching program on glaucoma medication adherence.18 All participants were adults with glaucoma who self-reported poor medication adherence at baseline. Their psychosocial characteristics were ascertained by survey and their glaucoma medication adherence was subsequently measured electronically for three months.
Participants and Sample Selection
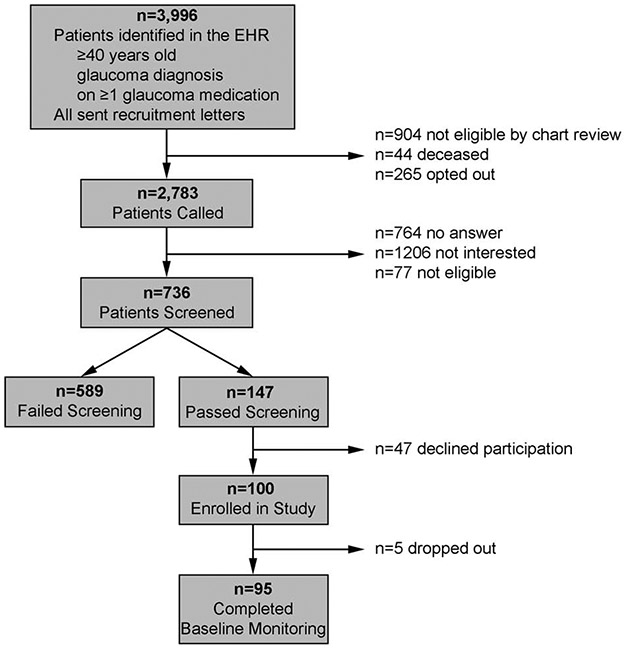
Participants were recruited between December 2016 and August 2018.To recruit glaucoma patients who were poorly adherent to their glaucoma medications, we used an automated data pull to identify participants who received primary or specialized ophthalmic care at the main or satellite University of Michigan Kellogg eye center clinics, had a diagnosis of glaucoma, were ≥40 age, and took ≥1 glaucoma medication. We then conducted a manual chart review to exclude individuals who were deceased, those with severe mental illness (defined as schizophrenia, bipolar disorder, or a major depressive episode with psychotic features), or cognitive impairment. We mailed letters to patients meeting the above mentioned criteria to enable patients to opt-out of receiving a recruitment phone call. A research associate called each patient who did not opt-out to ask if they would be interested in participating in a two-year study of a personalized glaucoma coaching program. If the patient was interested, the research associate obtained verbal consent to ask if the patient instilled their own eye drops and to administer two surveys to ascertain glaucoma medication adherence status. To be eligible for the study, participants had to speak English, instill their own eye drops and self-report poor adherence on the two validated scales, the Chang Adherence measure20 and the Morisky Medication Adherence Scale.21 To obtain the highest probability of capturing truly non-adherent patients, patients had to self-report poor adherence on both validated scales. Those who self-reported <95% adherence over the past month on the Chang measure and scored ≥2 on the Morisky scale were considered to have poor adherence by self-report. Participants who did not speak English were excluded from participation as the counseling program is delivered in English. The sample size estimates were calculated to assess the initial impact of the SEE program pilot study on glaucoma medication adherence. The pilot study required enrolling 46 participants with ≤80% adherence to provide 80% power to detect at least a relative improvement of 15% in adherence with a type 1 error of 5%. These estimates were based upon the work of Okeke et al.22 where a 20-percentage point increase in medication adherence was found after a personalized education program among participants with glaucoma with <75% adherence to their glaucoma medications. See Figure 1 for the flow diagram of participant recruitment and enrollment.
Figure 1.
Study Participant Flow Diagram
Once a potential participant met inclusion and exclusion criteria, including poor self-reported adherence, they were invited to participate in the SEE Program pilot study and a baseline study visit was scheduled. At the baseline study visit, written informed consent was obtained during which the process for ascertaining medication adherence through electronic monitoring was explained. This study was approved by the University of Michigan Institutional Review Board and followed all of the Tenets of the Declaration of Helsinki.
Survey and Adherence Measures
At the baseline study visit, participants completed a survey that incorporated four scales of psychological wellness validated in populations with chronic disease: perceived stress and resiliency, consideration of future consequences, glaucoma- related distress, and social support. The Cohen Perceived Stress Scale (PSS)23 measured perceived stress and resiliency. The PSS contains 10 items and is assessed on a scale from 0 to 40 (Cronbach’s α= 0.75-0.87).23 The Consideration of Future Consequences Scale (CFC) measured insight into how current behavior affects future status. The CFC contains 12 items and is assessed on a scale from 12 to 60 (Cronbach’s α=0.80-0.86).24 As no scale exists to assess specifically social support related to glaucoma, the Diabetes-Specific Social Support Needs Scale (SSNS) was adapted to glaucoma to assess perception of positive social support. The SSNS contains three items assessed on a scale from 3 to 21, where higher scores indicate increased social support (Cronbach’s α=0.52-0.69).25 Two additional questions ascertained awareness of glaucoma within a participant’s social network, asking whether or not the individual knows or knew someone with glaucoma and, if they do or did, whether that person had experienced vision loss (Appendix 1).
Disease-related distress is emotional distress resulting from troublesome symptoms, arduous self-management regimens, fear of complications, or loss of function from a chronic illness over and above perceived daily stress and underlying mental illness.26 The National Institute of Health has underscored the importance of creating programs to decrease diabetes-related distress given its impact on important diabetes outcomes, including glycemic control.9 We wanted to test whether disease related distress might also be associated with glaucoma self-management behaviors. In research about diabetes-related distress, worry about possible long-term complications and, ironically, a sense of anxiety or guilt surrounding the subject’s perception of poor-adherence were the items that most predicted poor diabetes medication adherence.27 No scale exists to specifically assess glaucoma-related distress. Because emotional burden and medication regimen were related to medication adherence in diabetes, we included items from the emotional-burden and regimen-related distress sub-scales in our assessment of glaucoma-related distress. We adapted a single question from the emotional burden sub-scale and two items from the regimen-related distress subscale from the Diabetes Distress Scale to glaucoma self-management.27 The three items we chose to assess glaucoma-related distress for our Glaucoma Distress Scale (GDS) were assessing whether people felt overwhelmed, angry, or like they were failing living with glaucoma. Each item on the GDS is assessed on a scale from 1 to 6, where higher numbers represent higher levels of distress.
Demographic information including income, education, race, ethnicity, and gender were ascertained from self-report. Functional health literacy was measured using the Functional Health Literacy Scale (3 item scale from 0-12, higher scale represents worse health literacy). 28 Data regarding mental health diagnoses, glaucoma severity, and best corrected visual acuity were extracted from the electronic medical record. Best corrected Snellen visual acuity was transformed to logMAR scale for analyses. Depression was further assessed for severity using the Patient Health Questionnaire (PHQ-9) scale (scale 0-27).29 The scale assesses severity of depression as follows: No depression, ≤9; Mild Depression, 10-14; Major Depression, Moderate, 15-19; Major Depression, Severe, ≥20.
Medication Adherence
Medication adherence was monitored using an electronic medication events monitoring system (MEMS, AdhereTech, New York, USA) for three months. Each glaucoma medication was placed into a MEMS bottle that looked like a large pill bottle. The bottle was labeled with the name of the medication and a color-coded sticker was placed around the bottle to correspond to the color of the glaucoma medication’s top. Each time the MEMS bottle was opened, the time and date stamp of this event was sent through the cellular data network to our secure database. Participants were aware that their adherence was being monitored.
An adherent event was defined as taking medication within a specified time window.18 For a once daily medication, an adherent event was defined as taking the medication within 24 ± 4 hours of the previous day’s dose. For a twice daily medication, an adherent event was defined as taking the first medication dose within 24 ± 2 hours of the previous day’s first dose, and taking the second medication dose within 24 ± 2 hours of the previous day’s second dose. For a three times daily medication, an adherent event was defined as taking the medication dose (first, second, or third) within 24 ± 1.3 hours of the previous day’s corresponding medication dose (first, second, or third). For a four times daily medication, an adherent event was defined as taking the medication dose (first, second, third, or fourth) within 24 ± 1 hour of the previous day’s corresponding medication dose. We included this time window because the biological efficacy of medications declines when not taken on time.30-32
When calculating adherence for medications dosed more than once a day, we compared the current day’s doses to the previous day’s doses rather than simply the previous dose (e.g., second versus first or third versus second) as lifestyle and sleeping patterns can result in medication times that are not equally spaced. This method of calculating adherence also allows for large shifts, such as changing shift times or going on vacation or gradual changes in times when medications are taken without overly penalizing the patient. Additionally, this method of measuring adherence ensures that times when a bottle is opened multiple times just prior to a clinic visit does not inflate the overall adherence metric.
For participants on more than one medication, adherence was first measured at the medication level and then aggregated to the person level by dividing the total number of doses of all medication(s) taken on time by the total number of doses of all medication(s) prescribed. Adherence was thus measured as a continuous variable on a scale from zero to one-hundred, each representing the percentage of prescribed glaucoma medications doses that were taken as scheduled. For each person, the adherence percentage was calculated monthly during the three-month monitoring period and the median of the three monthly percentages was used as the outcome variable “glaucoma medication adherence” for that person.
Statistical Analyses
Categorical variables were summarized by counts and percentages of non-missing values. Quantitative variables were summarized by means and standard deviations. Adherence was assessed monthly and the mean of the median adherence score was calculated. Histograms and boxplots were used to inspect and compare distributions. For responders with no missing items, scales from surveys were computed by summation, as recommended in the literature.27,33,34 For responders with missing items, the average of non-missing items was computed and then multiplied by the number of items on the scale. For each of the four psychological wellness scales, we assessed the internal consistency with Cronbach’s α.
Simple linear regressions were fit separately to quantify crude associations (via regression and/or correlation coefficients) of the primary outcome (glaucoma medication adherence) with psycho-social factors, demographics, and clinical variables. Inspection of regression diagnostics revealed no outliers or influential observations. Several multiple linear regression models were fit to investigate the importance of the numerous predictors. First, the regressions of adherence on each measure of psychological wellness were adjusted for income, a known strong confounder. Then, the association between GDS and adherence was explored after including all potential confounding variables from univariate analysis with p<0.1. The association between GDS and adherence was further expanded to assess other possible confounders using stepwise regression. To assess whether these modeling techniques lead to overfitting the model due to limited sample size, we fit a fourth multivariate regression model with Least Absolute Shrinkage and Selection Operator (LASSO) technique to identify the most significant potential confounding factors.34 Coefficients were interpreted as the average change in adherence associated with a one-unit change in the independent variable, all else held equal. Variance of adherence explained by its linear association with GDS was measured by partial R2. Multi-collinearity was assessed by variance inflation factors and was minimal.
Analyses were conducted in R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
147 individuals met inclusion and exclusion criteria for the study and had poor self-reported glaucoma medication adherence. 100 individuals consented to participate in the study (68% response rate). (Figure 1) Three months of electronic medication monitoring data and survey responses were obtained from 95 participants. Adherence to chronic ocular hypotensive therapy among study participants was a mean of 74% ± 21% (mean ± standard deviation, SD). (Table 1) The study population was 49% female, 97% had health insurance, and 63% had a college degree. The population was diverse with 35% African American participants and 8% Asian participants. Income was distributed fairly evenly in four categories (<$25,000 (19%), $25,000-$50,000 (28%), $51,000 - $100,000 (31%), and >$100,000 (22%)). Though nearly two-thirds of participants (64%) had 20/20 vision, glaucoma severity ranged from suspected glaucoma to severe glaucoma. While participants with severe mental illness were excluded from participation, more than one-third (37%) of participants had a diagnosis of depression, anxiety, and/or other less severe mental illness. Average PHQ-9 score was 3.1 ± 3.5. 94% of participants did not have depression (PHQ-9 score 0-9); 4% had Mild Depression (PHQ-9 score 10-14); 2% had Major Depression, Moderate (PHQ-9 score 15-19), and no one had Severe Major Depression (PHQ-9 score ≥20). (Table 1) Average Functional Health Literacy score was 1.9 ± 2.2.
Table 1:
Demographics and Clinical Data
| Frequency | Percentage | |
|---|---|---|
| All | 95 | 100.0% |
| Sex: Female | 47 | 49.5% |
| Race: | ||
| White | 52 | 54.7% |
| African American | 33 | 34.7% |
| Asian American | 8 | 8.4% |
| Other* | 2 | 2.1% |
| Ethnicity: | ||
| Non-Hispanic | 90 | 98.9% |
| Hispanic | 1 | 1.1% |
| missing | 4 | |
| Age: (yrs) | ||
| 40-59 | 32 | 33.7% |
| 60-69 | 34 | 35.8% |
| 70-88 | 28 | 29.5% |
| Income: ($000) | ||
| < 25 | 17 | 18.9% |
| 25-50 | 25 | 27.8% |
| 51-100 | 28 | 31.1% |
| > 100 | 20 | 22.2% |
| missing | 5 | |
| Insurance: No | 3 | 3.2% |
| Education: | ||
| Less than High School | 3 | 3.2% |
| High School Diploma | 9 | 9.5% |
| Some College | 23 | 24.2% |
| College Degree | 33 | 34.7% |
| Graduate Degree | 27 | 28.4% |
| Visual Acuity, better eye: | ||
| 20/20 | 61 | 64.2% |
| 20/21 - 20/29 | 20 | 21.1% |
| 20/30 or worse | 14 | 14.7% |
| Visual Acuity, worse eye: | ||
| 20/20 | 34 | 35.8% |
| 20/21 - 20/29 | 22 | 23.2% |
| 20/30 or worse | 39 | 41.1% |
| Glaucoma Severity, better eye: | ||
| Suspect** | 36 | 38.3% |
| Mild | 19 | 20.2% |
| Moderate | 16 | 17.0% |
| Severe | 23 | 24.5% |
| missing | 1 | |
| Glaucoma Severity, worse eye: | ||
| Suspect | 18 | 19.1% |
| Mild | 19 | 20.2% |
| Moderate | 16 | 17.0% |
| Severe | 41 | 43.6% |
| missing | 1 | |
| Any Mental Health Diagnosis: Yes | 35 | 36.8% |
| Depression Diagnosis: Yes | 30 | 31.6% |
| Anxiety Diagnosis: Yes | 13 | 13.7% |
| PHQ-9 Categories: | ||
| 0-9, No Depression | 88 | 93.6% |
| 10-14, Minor Depression | 4 | 4.3% |
| 15-19, Moderate Major | ||
| Depression | 2 | 2.1% |
| 20-27, Severe Major Depression | 0 | 0.0% |
| missing | 1 | |
| Adherence: | ||
| 0 - 40% | 6 | 6.3% |
| 41 - 60% | 16 | 16.8% |
| 61 - 80% | 31 | 32.6% |
| 81 - 100% | 42 | 44.2% |
“Other” denotes that the participant did not identify as White, African-American, or Asian.
The “Suspect” grouping includes those with a diagnosis of “glaucoma suspect” and those with a diagnosis of “ocular hypertension.”
Psychological Factors
The 95 participants’ glaucoma related distress as measured by GDS (Table 2) was relatively low (mean=5.6, sd=3.0 on a 3-18 scale). Item 2 (“[I feel] that I am often failing with my glaucoma routine”) had the highest mean (2.1, sd = 1.3, on a 1-6 scale). The CFC, measuring awareness of future consequences, had a mean of 43 (sd=8, on a 12-60 scale), the PSS, measuring perceived stress, had a mean of 12 (sd=7, on a 0-40 scale), and the SSNS, measuring perceived social support, had a mean of 13 (sd=5, on a 3-21 scale). To assess participants’ social networks, we found that most participants (78%) knew someone with glaucoma. Of those, 8% knew someone who had gone blind from glaucoma, 29% knew someone with lots of vision difficulty, 47% knew someone with some vision difficulty, and 15% knew someone with glaucoma who had maintained normal vision.
Table 2:
Summary of Four Scales and Responses to Items on Glaucoma Distress Scale
| Scale | Scale | N | mean | sd |
|---|---|---|---|---|
| Glaucoma Distress Scale (GDS) | 3-18 | 95 | 5.6 | 3.0 |
| GDS Q1 | 1-6 | 95 | 1.8 | 1.2 |
| GDS Q2 | 1-6 | 94 | 2.1 | 1.3 |
| GDS Q3 | 1-6 | 95 | 1.6 | 1.1 |
| Consideration of Future Consequences (CFC) | 12-60 | 95 | 43.4 | 8.0 |
| Perceived Stress Scale (PSS) | 0-30 | 95 | 12.3 | 6.5 |
| Social Support Needs Scale (SSNS) | 3-21 | 94 | 13.4 | 1.9 |
| Functional Health Literacy (FHL) | 3-15 | 95 | 1.9 | 2.2 |
| Patient Health Questionnaire (PHQ-9) | 0-27 | 94 | 3.1 | 3.5 |
| Question 1 | Question 2 | Question 3 | ||||
|---|---|---|---|---|---|---|
| GDS Item Responses | Freq | Pct | Freq | Pct | Freq | Pct |
| (1) Not a Problem | 53 | 55.8% | 40 | 42.6% | 61 | 64.2% |
| (2) A Slight Problem | 22 | 23.2% | 29 | 30.9% | 20 | 21.1% |
| (3) A Moderate Problem | 9 | 9.5% | 10 | 10.6% | 6 | 6.3% |
| (4) A Somewhat Serious Problem | 7 | 7.4% | 8 | 8.5% | 4 | 4.2% |
| (5) A Serious Problem | 2 | 2.1% | 5 | 5.3% | 3 | 3.2% |
| (6) A Very Serious Problem | 2 | 2.1% | 2 | 2.1% | 1 | 1.1% |
| missing | 0 | 1 | 0 | |||
Glaucoma Distress Scale (GDS) questions: “Thinking back to the past 4 weeks, how much, if any have the following problems bothered you? (Question 1) Feeling overwhelmed by the demands of living with my glaucoma; (Question 2) Feeling that I am often failing with my glaucoma routine; (Question 3) Feeling angry about having to live with glaucoma.”
Internal Consistency of the Survey Scales
To assess the internal consistency of the GDS, we calculated Cronbach’s α as 0.74 (95% CI 0.65, 0.83). For the SSNS in a glaucoma population, Cronbach’s α was 0.77 (95% CI 0.68, 0.85). For the PSS in our population, Cronbach’s α was 0.88 (95% CI 0.84, 0.92). Cronbach’s α for the CFC in our population was 0.85 (95% CI 0.80, 0.89).
Unadjusted Associations with Adherence
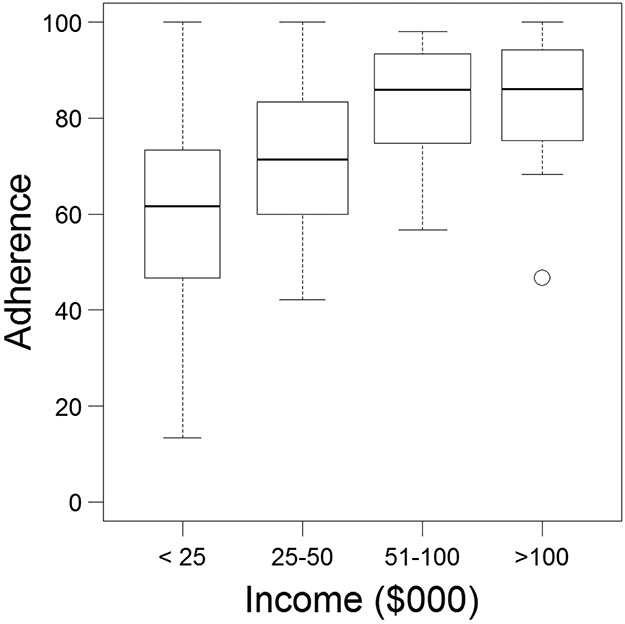
Glaucoma medication adherence was significantly associated with income and education. The higher the level of attained education, the higher the adherence (p=0.02). The higher the level of household income, the higher the level of medication adherence (p<0.0001). (Figure 2) Income accounted for approximately twice as much of the variability in medication adherence as education did (R2 education = 11.5%, R2 income = 25.4%). There were no significant associations between race and medication adherence (p=0.2). In terms of clinical factors, there were no significant associations between glaucoma medication adherence and severity of glaucoma in the better eye or the worse eye, better eye best-corrected vision or worse eye best-corrected vision. Though there was no significant association between diagnosed mental health conditions and glaucoma medication adherence, though there was a significant association between PHQ-9 score and glaucoma medication adherence. (R2 = 8.8, p = 0.004). (Table 3)
Figure 2.
Boxplot of the Relationship between Income and Glaucoma Medication Adherence
Table 3:
Univariate and Bivariate Associations between Medication Adherence and Glaucoma Related Distress, Consideration of Future Consequences, Perceived Stress, Social Support, Functional Health Literacy, and Depression (PHQ-9)
| Scale | Model | Coef | SE | p | R2 |
|---|---|---|---|---|---|
| Glaucoma Distress Scale (GDS) | crude | −3.32 | 0.65 | 0.0000 | 22.1 |
| adjusted | −2.41 | 0.63 | 0.0002 | 14.7 | |
| Consideration of Future Consequences (CFC) | crude | 0.77 | 0.26 | 0.0043 | 8.4 |
| adjusted | 0.34 | 0.25 | 0.1916 | 2.0 | |
| Perceived Stress Scale (PSS) | crude | −0.52 | 0.33 | 0.1191 | 2.6 |
| adjusted | −0.07 | 0.30 | 0.8241 | 0.1 | |
| Social Support Needs Scale (SSNS) | crude | −0.16 | 0.45 | 0.7174 | 0.1 |
| adjusted | 0.25 | 0.38 | 0.5155 | 0.5 | |
| Functional Health Literacy (FHL) | crude | −1.70 | 0.96 | 0.0805 | 3.2 |
| adjusted | −0.74 | 0.84 | 0.3819 | 0.9 | |
| Patient Health Questionnaire (PHQ-9) | crude | −1.79 | 0.60 | 0.0037 | 8.8 |
| adjusted | −1.14 | 0.52 | 0.0316 | 5.4 |
Adjusted models are adjusted for income.
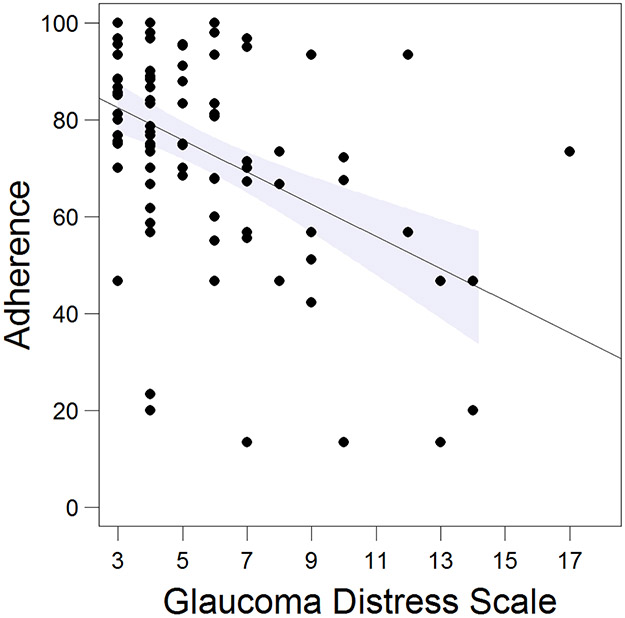
In terms of psychological factors, the more importance people placed on future consequences as measured by a higher CFC score, the higher the adherence score (p=0.004, Table 3). The higher the amount of glaucoma-related distress as measured by a higher GDS score, the worse the adherence level (p < 0.0001, Figure 3, Table 3). Glaucoma-related distress accounted for 22% of the variance of glaucoma medication adherence in univariate analysis. We assessed the association between each individual glaucoma-related distress item and glaucoma medication adherence. “Feeling overwhelmed by the demands of living with my glaucoma” accounted for 11% of the variance in glaucoma medication adherence (R2= 11%, p=0.0009). “Feeling that I am often failing with my glaucoma routine” accounted for 37% of the variance in glaucoma medication adherence (R2= 37%, p<0.0001). “Feeling angry about having to live with glaucoma” accounted for 4% of the variance in glaucoma medication adherence (R2= 4%, p=0.04). There were no significant associations between perceived stress, social support, or social network on adherence to glaucoma medications.
Figure 3.
Relationship between Glaucoma Related Distress and Glaucoma Medication Adherence
Adjusted Associations with Adherence
After adjusting for income, glaucoma related distress remained a significant predictor of glaucoma medication adherence (p=0.0002, Table 3) while consideration of future consequences did not (p=0.2, Table 3). We included all covariates that had a univariate association with glaucoma medication adherence at the level of p<0.1 (GDS, income, education, age, PHQ-9 score, FHL, and CFC), the relationship between glaucoma related distress and medication adherence remained statistically significant (p=0.004, Table 4). When we used the stepwise regression approach to choose covariates for the multivariate model, GDS, income, CFC, age and sex were included as covariates and the relationship between glaucoma related distress and medication adherence remained significant (p=0.0016, Table 4). Because both of these models chose different covariates, and the standard error for the magnitude of the association between glaucoma related distress and medication adherence increased from 0.6 in the crude model to 2.1 in the first model and 1.9 in the stepwise regression model, we were concerned that the models may be overfit given our limited sample size. Using the LASSO technique selected only GDS and a low-income indicator as covariates in the model and gave results between the model adjusted only for income (a beta coefficient of −4.8 compared to a beta coefficient of −2.4 in the income adjusted model and −6.3 in the stepwise model). Therefore, we will discuss further the results from the income adjusted multivariate model, which is a more conservative estimate of the effect size than the other models. In the income adjusted model, every one-point increase on the 18-point glaucoma-related distress scale was associated with a 2.4 percentage point decrease in glaucoma medication adherence (p=0.0002, Table 4). This multivariate model predicted 36% of the variance in glaucoma medication adherence. The partial R2 for glaucoma related distress in this model was 15%, meaning that after adjusting for the significant confounder of income, glaucoma related-distress accounted for 15% of the variance in medication adherence. The partial R2 for income was also 15%.
Table 4:
Multivariate Models of the Association of Glaucoma Related Distress and Glaucoma Medication Adherence
| Variable | Crude | Model | Stepwiseb | |
|---|---|---|---|---|
| Income Adjusted |
Univariate Screena | |||
| Glaucoma Distress Scale, p | <0.0001 | 0.0002 | 0.0038 | 0.0016 |
| coefficient (se) | −3.3 (0.6) | −2.4 (0.6) | −6.4 (2.1) | −6.3 (1.9) |
| Income, p | 0.0031 | 0.0672 | 0.0109 | |
| (0) <25K, coefficient (se) | <ref> | <ref> | <ref> | |
| (1) 25-50K, coefficient (se) | 11.5 (5.0) | 7.3 (4.9) | 6.8 (4.9) | |
| (2) 51-100K, coefficient (se) | 19.0 (5.2) | 14.1 (5.2) | 16.3 (5.0) | |
| (3) >100K, coefficient (se) | 18.0 (5.6) | 12.2 (6.1) | 15.4 (5.7) | |
| PHQ9, p | 0.5313 | |||
| coefficient (se) | −0.32 (0.51) | |||
| FHL, p | 0.8841 | |||
| coefficient (se) | −0.12 (0.84) | |||
| CFC, p | 0.0665 | 0.0778 | ||
| coefficient (se) | 0.44 (0.23) | 0.41 (0.23) | ||
| Age, p | 0.0277 | 0.0024 | ||
| coefficient (se) | 0.40 (0.18) | 0.50 (0.16) | ||
| Sex, p | 0.0202 | |||
| Male, coefficient (se) | −7.7 (3.2) | |||
| Education, p | 0.5702 | |||
| (1) HS Diploma or Less, coefficient (se) | <ref> | |||
| (2) Some College, coefficient (se) | −6.5 (5.8) | |||
| (3) College Diploma, coefficient (se) | −2.6 (5.7) | |||
| (4) Graduate School, coefficient (se) | −0.6 (6.1) | |||
| Model R^2 | 22.1% | 36.4% | 48.7% | 50.8% |
| Partial R^2 for GDS | 22.1% | 14.7% | 10.4% | 12.2% |
| Partial R^2 for Income | 14.9% | 8.8% | 13.4% | |
Model includes all variables having p<0.1 in univariate model for adherence.
Stepwise regression by Akaike Information Criterion starting with GDS only model.
Abbreviations: p, p value; se, standard error; PHQ-9, Patient Health Questionnaire-9; FHL, Functional Health Literacy; CFC, Consideration of Future Consequences; HS, High School; GDS, Glaucoma Distress Scale
Discussion
In this study we explored the relationship between electronically monitored glaucoma medication adherence and the following baseline SEE Program pilot study participant factors: demographics, glaucoma severity, perceived stress, disease-related distress, social support, and insight into future consequences. Lower levels of income and education both significantly predicted worse medication adherence. Glaucoma severity, mental health diagnosis and race did not predict medication adherence in our sample. Of the four psycho-social factors we assessed, only glaucoma-related distress was found to have a statistically significant association with medication adherence after adjustment for confounding variables. In univariate analysis, those who put less weight on future consequences were more likely to have poor medication adherence. After adjusting for income, this association was no longer significant. Our study results demonstrate that a higher level of glaucoma-related distress due to perceived disease burden is associated with lower adherence to a chronic glaucoma medication regimen, independent of socio-demographic factors.
This investigation is the first to establish a relationship between glaucoma-related distress and adherence to chronic ocular hypotensive therapy. In this study, glaucoma-related distress was measured by the Glaucoma Distress Scale, a preliminary adaptation of the Diabetes Distress Scale for glaucoma. After adjusting for potential confounders in a multivariate analysis, for every point increase in glaucoma related distress on a 15-point scale (from 3 to 18), we saw a 2.4 percentage point decrease in medication adherence. This means that a person who reported a glaucoma related distress score of 13 would have approximately 24 percentage point worse medication adherence compared to someone who reported a glaucoma related distress score of 3. The mean glaucoma distress scale score in this cohort was 5.6 ±3.0, so a person reporting a level of glaucoma-related distress one standard deviation above the mean would have 7.2 percentage points worse adherence than a participant reporting the mean level of glaucoma-related distress. We found that the three items we used from these two sub-scales, and particularly the item from the regimen-related distress subscale, were significantly associated with poor medication adherence. The question about regimen-related distress, “Feeling that I am often failing with my glaucoma routine,” was highly correlated with the median monthly adherence from three subsequent months of electronic monitoring (crude R2 = 37%, p<0.0001). The strength and magnitude of the relationship between a preliminary assessment of glaucoma related distress and medication adherence underscores the next important step in this research trajectory: developing a validated scale to use as a patient-centered outcome measure to assess glaucoma-related distress. This will require a larger sample of glaucoma patients with varying levels of medication adherence so that the results generalize better to the glaucoma population as a whole.
Our result that glaucoma-related distress, independent of mental illness, is associated with adherence is a finding that mirrors that of previous studies in the diabetes literature. Multiple prior studies have identified that diabetes disease-related distress has an impact on health outcomes even after adjusting for co-morbid mental illness. Two separate groups found that diabetes-related distress was associated with poor diabetes medication adherence after adjusting for both the presence of and severity of depression.35,36In one study, a higher level of diabetes-related distress was significantly associated with a higher hemoglobin A1C (HbA1c) value, where a ten-point increase in diabetes-related distress was related to a 2.1 point increase in HbA1c, a large clinical impact. In contrast, depression severity was not significantly related to HbA1C. These findings suggest that disease-related distress has an independent impact on health outcomes in addition to co-morbid depression. Both disease-related distress and depression should be assessed and addressed to improve chronic disease self-management both among patients with diabetes and among patients with glaucoma.
Importantly, we also found that income was a very important driver of medication adherence, accounting for 15% of the variance in medication adherence in a model adjusting for the impact of glaucoma related distress. There is a robust literature that demonstrates the impact of low income on health outcomes. Our finding that people with lower household incomes were significantly more likely to have poor medication adherence echo this. Cost is a significant barrier to medication adherence for those with many chronic medical conditions and may also be a significant barrier in our population, even though 97% of our sample population had health insurance.36,37 In 2016, Raj and colleagues studied income data from de-identified tax records and mortality records from the social security administration and generated life expectancy predictions for 40 year-olds in the US. They found stark disparities in mortality by household net income – life expectancy was 4.5 years lower in those in the lowest income quartile compared to those in the highest income quartile.38 In the United States, poor adults are five times as likely to report being in poor or fair health compared to those who have income 400 percent above the federal poverty level.39 Similarly, lower educational attainment has a large impact on overall health status. An additional four years of education lowers five-year mortality by 1.8 percentage points; reduces the risk of heart disease by 2.16 percentage points, and lowers the risk of diabetes by 1.3 percentage points. 40 Poverty and lower education may contribute to poor medication adherence and poor overall health in myriad ways over and above simply the cost of the medication. Limited resources and limited education make it difficult for people to choose between day-to-day necessities and medication. People with lower income also have to prioritize which medications they “really can’t miss.” 41 Accessing transportation to the doctor and pharmacy can be a challenge. Scheduling in medical care and visits to the pharmacy can also be challenging if people’s work schedules are not regular or their work has limited flexibility.42 Understanding the importance of managing a chronic disease can also be impacted by educational attainment.43 Poverty and low educational attainment play a significant role in mediating people’s ability to optimally self-manage their glaucoma.
We hypothesized that social support, social network, perceived stress, and future orientation would all be associated with glaucoma medication adherence, but did not find these associations in this sample. In the univariate analysis, we found that CFC was related to adherence. CFC was highly correlated with income as when we adjusted for income alone, the association between CFC and medication adherence was no longer significant. It may be that those with lower income are less able to focus on future consequences as they are struggling to stay afloat day-to-day.44
Many previous studies have established race as a predictor of poor adherence, identifying African-American patients as those least likely to adhere to chronic medical regimens, after adjusting for economic status and education.45-48 We did not find race to be a significant predictor of medication adherence in our sample. It is possible that previous studies did not sample a sufficiently diverse sample to tease apart the impact of race, income and education on medication adherence. The majority of participants in our study sample had a college degree or graduate degree. The average personal income was nearly twice that of the national average. We hypothesize that because our study participants were largely well-educated and of a higher socioeconomic status, the impact that was attributed to race in previous studies was not found in our study.
We also hypothesized that positive social support would be associated with higher levels of adherence because social support has consistently been linked to better health outcomes among adults with chronic conditions. For example, a meta-analysis of fifty studies found that practical social support was most consistently associated with greater medication adherence.49 A study of individuals with type 2 diabetes drew from both these models and found that support systems improve health outcomes among those with chronic conditions by ameliorating the effect of disease related distress.50 However, we did not find a significant association between social support and adherence, or a moderating effect of social support on the relationship between disease related distress and adherence. This may be due to our limited sample size or due to the instrument used to measure social support, which we adapted from the diabetes literature for the glaucoma population.
The strengths of this study lie in the diversity of the sample and the accuracy with which adherence was measured. Electronically monitoring glaucoma medication adherence is the gold standard in adherence assessment. However, this study also has a number of limitations. The use of electronic medication monitoring, thought the current gold standard, is still limited as participants may open the container and not take the medication, forget to place the bottle of medication back into the electronic monitor, take an incorrect number of drops, or take the medication but not get the drop into the eye. Because we were assessing baseline psycho-social characteristics of participants enrolled in a two-year pilot study of a personalized glaucoma coaching program and all participants had to have poor self-reported medication adherence to enroll, the population is not reflective of the general population with glaucoma. Had the study included participants with a wider range of glaucoma medication adherence, we might have found an association with glaucoma severity and mental health diagnosis. In addition, 97% of participants had health insurance and 63% had a college degree, which further limits the generalizability of the findings. We excluded participants with severe mental illness. Had this not been an exclusion criterion, we may have seen an association between mental illness and medication adherence. The lack of a validated instrument to capture glaucoma related distress may have limited our analysis. In addition, because the scale was originally developed to assess diabetes related distress and not glaucoma related distress, there may be issues with construct validity such that participants may not have interpreted the items such as “Feeling that I am often failing with my glaucoma routine" as a question assessing their emotional burden but may have interpreted as a direct assessment of how well they are taking their medications. Because of the identified importance of this construct, future work will focus on developing and validating a glaucoma related distress scale. Further, the association presented between glaucoma related distress and medication adherence is non-causal; further prospective research using a randomized controlled trial design would better establish this relationship. The lack of a validated instrument to measure social support may have limited our analysis of this important construct as well.
Our study suggests that glaucoma-related distress is an important patient-centered outcome measure to help identify populations at risk for poor adherence. Our findings suggest that assessing patients’ glaucoma related distress using a brief screen – as many practices do now for diabetes-related distress—and providing these patients with increased self-management support may improve people’s overall experience with their glaucoma care. Creating interventions to decrease glaucoma-related distress may also improve medication adherence among glaucoma patients. Decreasing glaucoma-related distress may improve the quality of glaucoma care and clinical outcomes.
Acknowledgements
Funding/Support: National Eye Institute (K23EY025320, PANC) and Research to Prevent Blindness Career Development Award (PANC). The funding organizations had no role in the design or conduct of this research.
Funding Agency: National Eye Institute (K23EY025320, PANC) and Research to Prevent Blindness Career Development Award (PANC). The funding organizations had no role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: No financial disclosures exist for any author.
This work was presented in part at: The American Glaucoma Society Annual Meeting, San Francisco, CA, March 16, 2019
Conflict of Interest: No conflicting relationships exist for any author.
REFERENCES
- 1.Cutler DM, Everett W. Thinking Outside the Pillbox — Medication Adherence as a Priority for Health Care Reform. N Engl J Med. 2010;362:1553–1555. [DOI] [PubMed] [Google Scholar]
- 2.Stewart K, George J, Mc Namara KP, et al. A multifaceted pharmacist intervention to improve antihypertensive adherence: a cluster-randomized, controlled trial (HAPPy trial). J Clin Pharm Ther. 2014;39:527–534. [DOI] [PubMed] [Google Scholar]
- 3.Presley B, Groot W, Pavlova M. Pharmacy-led interventions to improve medication adherence among adults with diabetes: A systematic review and meta-analysis. Res Soc Adm Pharm. 2019;15:1057–1067. [DOI] [PubMed] [Google Scholar]
- 4.van Driel ML, Morledge MD, Ulep R, Shaffer JP, Davies P, Deichmann R. Interventions to improve adherence to lipid-lowering medication. Cochrane Database Syst Rev. 2016;12:CD004371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuller RH, Perel P, Navarro-Ruan T, Nieuwlaat R, Haynes RB, Huffman MD. Improving medication adherence in patients with cardiovascular disease: a systematic review. Heart. 2018;104:1238–1243. [DOI] [PubMed] [Google Scholar]
- 6.Leske MC. Factors for Glaucoma Progression and the Effect of Treatment. Arch Ophthalmol. 2003;121:48–56. [DOI] [PubMed] [Google Scholar]
- 7.Garway-Heath DF, Crabb DP, Bunce C, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;385:1295–1304. [DOI] [PubMed] [Google Scholar]
- 8.Rossi GCM, Pasinetti GM, Scudeller L, Radaelli R, Bianchi PE. Do Adherence Rates and Glaucomatous Visual Field Progression Correlate? Eur J Ophthalmol. 2011;21:410–414. [DOI] [PubMed] [Google Scholar]
- 9.Miller T, DiMatteo R. Importance of family/social support and impact on adherence to diabetic therapy. Diabetes, Metab Syndr Obes Targets Ther. 2013;2013:421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee AA, Piette JD, Heisler M, Rosland A-M. Diabetes Distress and Glycemic Control: The Buffering Effect of Autonomy Support From Important Family Members and Friends. Diabetes Care. 2018;41:1157–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ajit RR, Fenerty CH, Henson DB. Patterns and rate of adherence to glaucoma therapy using an electronic dosing aid. Eye. 2010;24:1338–1343. [DOI] [PubMed] [Google Scholar]
- 12.Sleath B, Blalock S, Covert D, et al. The Relationship between Glaucoma Medication Adherence, Eye Drop Technique, and Visual Field Defect Severity. Ophthalmology. 2011;118:2398–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerlach LB, Kavanagh J, Watkins D, Chiang C, Kim HM, Kales HC. With a little help from my friends?: racial and gender differences in the role of social support in later-life depression medication adherence. Int Psychogeriatrics. 2017;29:1485–1493. [DOI] [PubMed] [Google Scholar]
- 14.Scheurer D, Choudhry N, Swanton KA, Matlin O, Shrank W. Association between different types of social support and medication adherence. Am J Manag Care. 2012;18:e461–467. [PubMed] [Google Scholar]
- 15.Dreer LE, Girkin C, Mansberger SL. Determinants of Medication Adherence to Topical Glaucoma Therapy. J Glaucoma. 2012;21:234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen S Psychosocial models of the role of social support in the etiology of physical disease. Heal Psychol. 1988;7:269–297. [DOI] [PubMed] [Google Scholar]
- 17.Newman-Casey PA, Shtein RM, Coleman AL, Herndon L, Lee PP. Why Patients With Glaucoma Lose Vision. J Glaucoma. 2016;25:e668–e675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newman-Casey PA, Niziol LM, Mackenzie CK, et al. Personalized behavior change program for glaucoma patients with poor adherence: a pilot interventional cohort study with a pre-post design. Pilot Feasibility Stud. 2018;4:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strom JL, Egede LE. The Impact of Social Support on Outcomes in Adult Patients with Type 2 Diabetes: A Systematic Review. Curr Diab Rep. 2012;12:769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang DS, Friedman DS, Frazier T, Plyler R, Boland MV. Development and Validation of a Predictive Model for Nonadherence with Once-Daily Glaucoma Medications. Ophthalmology. 2013;120:1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morisky DE, Green LW, Levine DM. Concurrent and Predictive Validity of a Self-reported Measure of Medication Adherence. Med Care. 1986;24:67–74. [DOI] [PubMed] [Google Scholar]
- 22.Okeke CO, Quigley HA, Jampel HD, et al. Interventions Improve Poor Adherence with Once Daily Glaucoma Medications in Electronically Monitored Patients. Ophthalmology. 2009; 116:2286–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee E-H. Review of the Psychometric Evidence of the Perceived Stress Scale. Asian Nurs Res (Korean Soc Nurs Sci). 2012;6:121–127. [DOI] [PubMed] [Google Scholar]
- 24.Yanover T, Sacco WP. Reliability of diabetes-specific social support scales. Psychol Health Med. 2008;13:627–631. [DOI] [PubMed] [Google Scholar]
- 25.Rappange DR, Brouwer WBF, van Exel NJA. Back to the Consideration of Future Consequences Scale: Time to Reconsider? J Soc Psychol. 2009;149:562–584. [DOI] [PubMed] [Google Scholar]
- 26.Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabet Med. 2014;31:764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polonsky WH, Fisher L, Earles J, et al. Assessing Psychosocial Distress in Diabetes: Development of the Diabetes Distress Scale. Diabetes Care. 2005;28:626–631. [DOI] [PubMed] [Google Scholar]
- 28.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36:588–594. [PubMed] [Google Scholar]
- 29.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. J Gen Intern Med. 2001;16:606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhagav P, Trivedi V, Shah D, Chandran S. Sustained release ocular inserts of brimonidine tartrate for better treatment in open-angle glaucoma. Drug Deliv Transl Res. 2011;1:161–174. [DOI] [PubMed] [Google Scholar]
- 31.Loftsson T, Jansook P, Stefánsson E. Topical drug delivery to the eye: dorzolamide. Acta Ophthalmol. 2012;90:603–608. [DOI] [PubMed] [Google Scholar]
- 32.Ellis PP, Wu PY, Riegel M. Aqueous humor pilocarpine and timolol levels after instillation of the single drug or in combination. Investig Ophthalmol Vis Sci. 1991;32:520–522. [PubMed] [Google Scholar]
- 33.Cohen S Perceived Stress Scale. https://www.mindgarden.com/132-perceived-stress-scale#horizontalTab1. Accessed 1/14/2020
- 34.Strathman A, Gleicher F, Boninger DS, Edwards CS. The consideration of future consequences: Weighing immediate and distant outcomes of behavior. J Pers Soc Psychol. 1994;66:742–752. [Google Scholar]
- 35.Lee T-F, Chao P-J, Ting H-M, et al. Using Multivariate Regression Model with Least Absolute Shrinkage and Selection Operator (LASSO) to Predict the Incidence of Xerostomia after Intensity-Modulated Radiotherapy for Head and Neck Cancer Cordes N, ed. PLoS One. 2014;9:e89700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes Distress but Not Clinical Depression or Depressive Symptoms Is Associated With Glycemic Control in Both Cross-Sectional and Longitudinal Analyses. Diabetes Care. 2010;33:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaleva V Adherence to medication. Pediatriya. 2015;55:68–69. [Google Scholar]
- 38.Mishra SI, Gioia D, Childress S, Barnet B, Webster RL. Adherence to Medication Regimens among Low-Income Patients with Multiple Comorbid Chronic Conditions. Health Soc Work. 2011;36:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chetty R, Stepner M, Abraham S, et al. The Association Between Income and Life Expectancy in the United States, 2001-2014. JAMA. 2016;315:1750–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Picker L The Effects of Education on Health. https://www.nber.org/digest/mar07/w12352.html. Accessed 1/14/2020
- 41.Braveman P, Egerter S. Overcoming Obstacles to Health - RWJF. https://www.rwjf.org/en/library/research/2008/02/overcoming-obstacles-to-health.html. Accessed 1/14/2020
- 42.Henry J KFF. Seniors and Prescription Drugs: An 8-State Survey. The Henry J. Kaiser Family Foundation; https://www.kff.org/medicare/report/seniors-and-prescription-drugs-an-8-state/. Accessed 1/14/2020 [Google Scholar]
- 43.Vélez-Gómez MC, Vásquez-Trespalacios EM. Adherence to topical treatment of glaucoma, risk and protective factors: A review. Arch Soc Esp Oftalmol. 2018;93:87–92. [DOI] [PubMed] [Google Scholar]
- 44.Woodward MA, Jeganathan VSE, Guo W, Cederna J, Newman-Casey PA. Barriers to Attending Eye Appointments among Underserved Adults. J Ophthalmic Vis Res. 2017;12:449–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robin AL, Novack GD, Covert DW, Crockett RS, Marcic TS. Adherence in Glaucoma: Objective Measurements of Once-Daily and Adjunctive Medication Use. Am J Ophthalmol. 2007;144:533–540.e2. [DOI] [PubMed] [Google Scholar]
- 46.Pandit AU, Bailey SC, Curtis LM, et al. Disease-related distress, self-care and clinical outcomes among low-income patients with diabetes. J Epidemiol Community Health. 2014;68:557–564. [DOI] [PubMed] [Google Scholar]
- 47.Lee M, Salloum RG. Racial and ethnic disparities in cost-related medication non-adherence among cancer survivors. J Cancer Surviv. 2016;10:534–544. [DOI] [PubMed] [Google Scholar]
- 48.Sleath B, Blalock SJ, Covert D, Skinner AC, Muir KW, Robin AL. Patient Race, Reported Problems in Using Glaucoma Medications, and Adherence. ISRN Ophthalmol. 2012;2012:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.ROBIN A, COVERT D. Does Adjunctive Glaucoma Therapy Affect Adherence to the Initial Primary Therapy? Ophthalmology. 2005;112:863–868. [DOI] [PubMed] [Google Scholar]
- 50.Tsai JC, McClure CA, Ramos SE, Schlundt DG, Pichert JW. Compliance barriers in glaucoma: a systematic classification. J Glaucoma. 2003;12:393–398. [DOI] [PubMed] [Google Scholar]