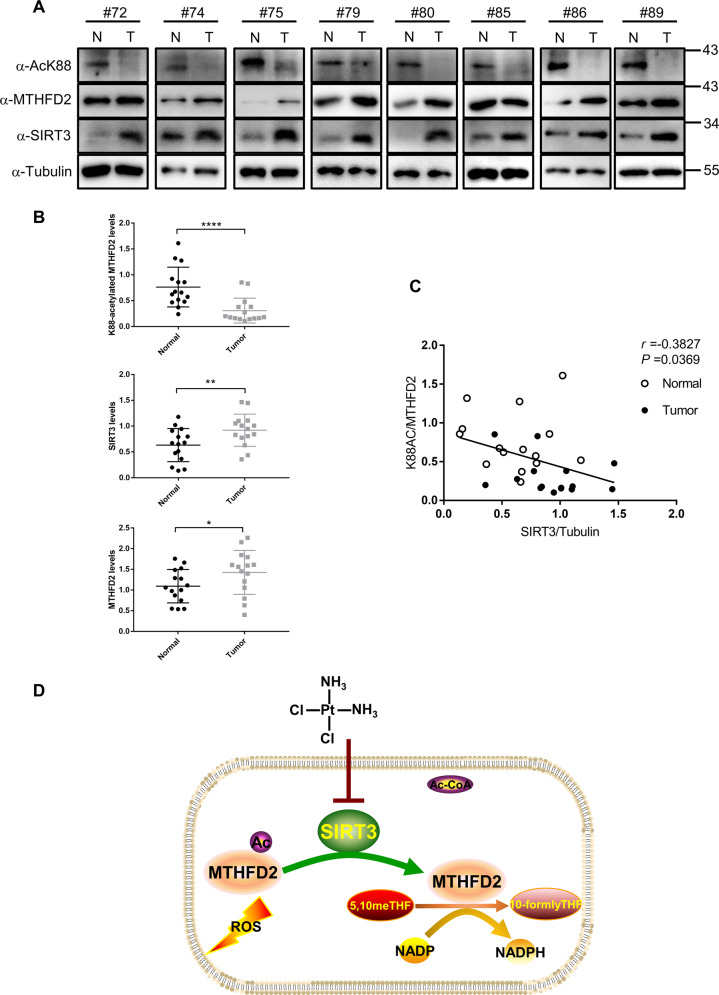
Fig. 6. K88 acetylation of MTHFD2 is downregulated in CRC.
a In total, 15 pairs of tumor tissues (T) and adjacent normal tissues (N) were lysed. Protein levels of MTHFD2-K88Ac, MTHFD2, and SIRT3 were determined by direct western blot. Relative protein levels were normalized by α-Tubulin. Shown are eight pairs of samples. b Quantification of relative MTHFD2, MTHFD2-K88Ac and SIRT3 protein levels in the 15 pairs of samples tested. The intensities of indicated proteins were quantified using the ImageJ software, followed by statistical analysis. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; n.s., not significant for the indicated comparison. c SIRT3 protein levels show negative correlation with MTHFD2-K88Ac. Correlation between MTHFD2 protein levels and MTHFD2-K88Ac levels in the tested 15 pairs of samples. Statistical analyses were performed with F test. d Working model. Acetylation at K88 under cisplatin treatment decreases SIRT3 expression, increases MTHFD2 acetylation level and inhibits MTHFD2 enzyme activity. In CRC, high SIRT3 levels promotes deacetylation of MTHFD2 to maintain its high activity and restraining cellular redox balance.