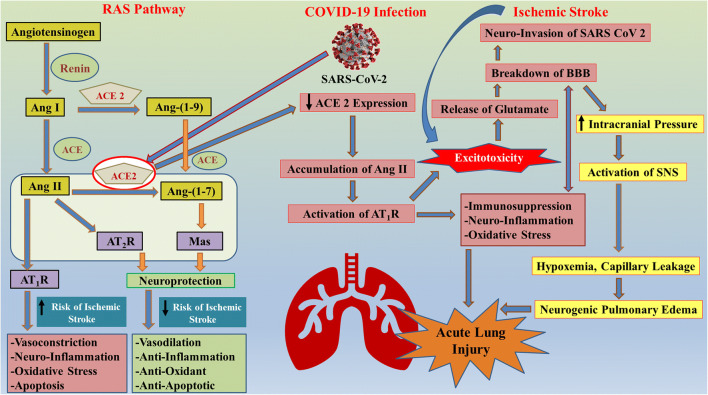
Fig. 2.
Schematic diagram illustrating the role of RAS in cross-talk between ischemic stroke and SARS-CoV-2 infection. The brain RAS is regulator of physiological homeostasis and cerebrovascular disorders such as IS. Classical RAS elements include ACE, Ang II, and AT1R. Angiotensinogen is hydrolyzed to Ang I by the aspartyl protease renin enzyme. Furthermore, ACE hydrolyzes the Ang I into Ang II. The interaction of newly formed Ang II with AT1R increases the risk of ischemic stroke as it results in an enhanced risk of thrombosis. On the other hand, the newly found RAS pathway exhibits the hydrolysis of Ang II in the presence of ACE2 to Ang-(1–7), which acts as a ligand for the Mas receptor. This new axis has anti-oxidative, anti-inflammatory, and antithrombotic activity, thus decreasing the risk of ischemic stroke. The binding of SARS-CoV-2 with ACE2 receptor in lungs leads to the downregulation of ACE2 expression, which further results in accumulation in Ang II, thus activating the AT1R pathway and increased risk of IS. AT1R pathway activation leads to excitotoxicity, immunosuppression, neuroinflammation, and oxidative stress, finally resulting in acute lung injury. A secondary cascade is also activated due to excitotoxicity which results in the release of stored glutamate in brain cells, thus disrupting the BBB and allowing easy access of SARS-CoV-2 in brain. Breakdown of BBB also increases the intracranial pressure resulting in activation of SNS, hypoxia, capillary leakage, neurogenic pulmonary edema, and finally acute lung injury. Excitotoxicity is a crucial link between COVID-19 and IS, as incidence of IS also results in excitotoxicity and breakdown of BBB, resulting in higher risk of comorbidity. ↓ Decrease; ↑ Increase