Figure 3.
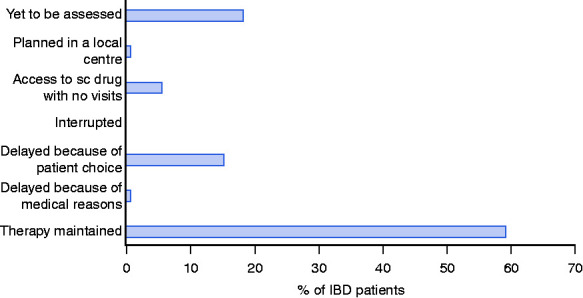
Patients under biological and experimental therapy management after COVID-19 pandemic at the IBD centre. Absolute numbers (and percentages) of observed cases over the general IBD population receiving biological or clinical trial therapy are shown. Out of 1451 patients, 266 (18.3%) were yet to be assessed, 11 (0.77%) were planned in a local centre, 82 (5.62%) had access to subcutaneous drugs with no follow-up visit, 0 (0%) were interrupted, 222 (15.33%) were delayed because of patient's choice, 11 (0.77%) were delayed because for medical reasons and 859 (59.2%) had their therapy maintained.
