Figure 4.
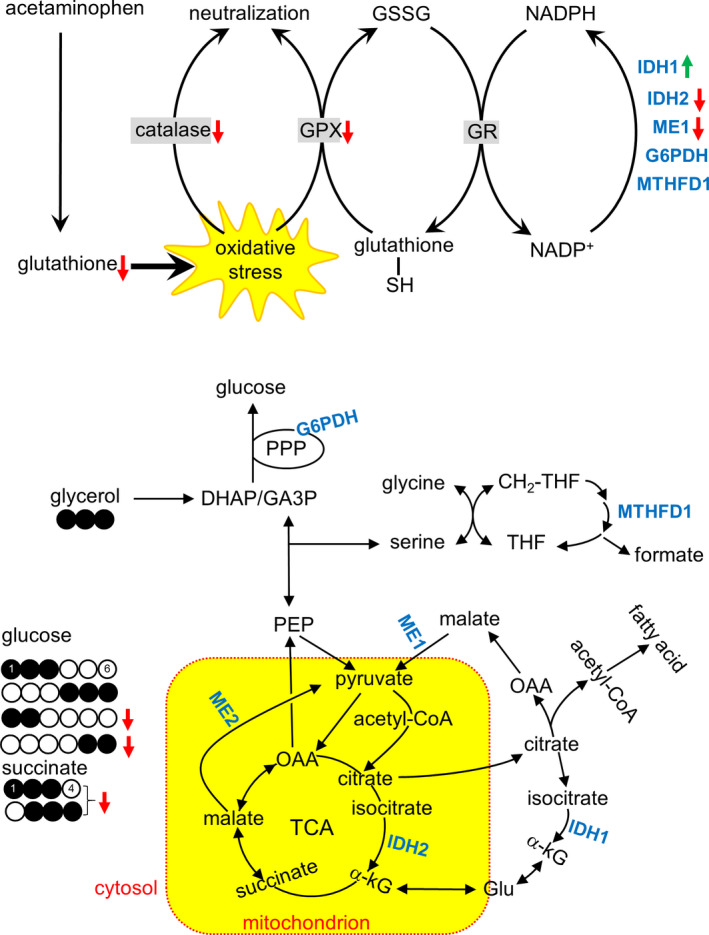
Illustration of the effects of acetaminophen on glutathione, antioxidant processes, and metabolic processes. Acetaminophen depleted glutathione in the liver causing oxidative stress. It also reduced the activities of catalase and glutathione peroxidase (GPX) that neutralize oxygen radicals, but did not change the activity of glutathione reductase (GR) that requires NADPH to regenerate reduced glutathione. Responses of NADPH‐producing enzymes were complex; increased isocitrate dehydrogenase 1 (IDH1), decreased IDH2 and malic enzyme 1 (ME1), and unchanged glucose 6‐phosphate dehydrogenase (G6PDH) and methylenetetrahydrofolate dehydrogenase 1 (MTHFD1). According to NMR analysis of glucose, the production of triple‐labeled ([1,2,3‐13C3] and [4,5,6‐13C3]) glucose remained unchanged with acetaminophen treatment, informing intact gluconeogenesis directly from [U‐13C3]glycerol in the cytosol. However, both [1,2‐13C2]glucose produced through the PPP and [5,6‐13C2]glucose produced through the TCA cycle were suppressed. In NMR analysis of liver extracts, triple‐labeled ([1,2,3‐13C3] and [2,3,4‐13C3]) succinate was reduced with acetaminophen, informing suppressed pyruvate carboxylation to enter the TCA cycle. Abbreviation: DHAP, dihydroxyacetone phosphate; GA3P, glyceraldehyde 3‐phosphate; Glu, glutamate; GSSG, glutathione disulfide; α‐kG, alpha‐ketoglutarate; OAA, oxaloacetate; PEP, phosphoenolpyruvate; THF, tetrahydrofolate
