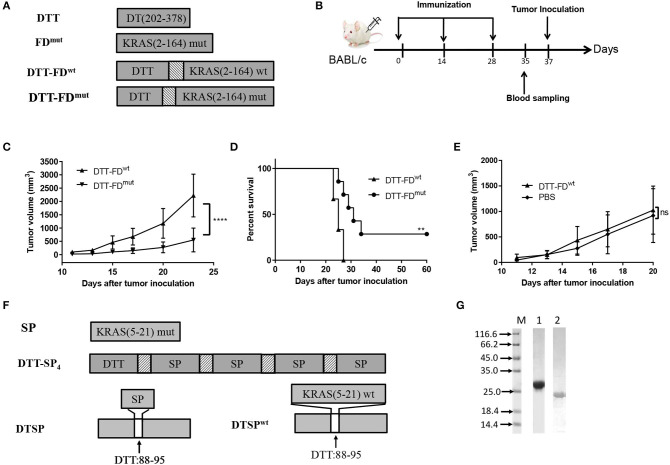
Figure 1.
Rationally designed mutant peptide-based vaccines. (A–E) Contributions of mutated and non-mutated epitopes to Anti-Tumor efficacy were determined when DTT was used as a carrier protein. (A) DTT is the diphtheria toxin T-domain, corresponding to amino acids 202–378 of DT. FDmut represents KRAS G domain (residues 2–164) carrying the G12D mutation. DTT-FDmut and DTT-FDwt were constructed by fusing KRAS G domain (residues 2–164) to the C-terminal of DTT through a GS linker. DTT-FDmut and DTT-FDwt represent presence and absence of KRAS G12D mutation, respectively, in the fusion construct. (B) Flow chart of immunization and tumor inoculation. (C–E) DTT-FDmut, DTT-FDwt, and PBS were separately formulated in Alum and CpG. Female BALB/c mice (n = 5–8) received the formulated vaccines three times at 2-week intervals. Mice were injected with 2 × 105 cells/mouse 10 days after the last immunization. (C) Tumor growth curves of DTT-FDmut and DTT-FDwt were plotted by averaging tumor size over time in each group. Data are presented as means ± SD. ****p < 0.0001, Student's t-test (D) Kaplan–Meier survival curve. **p < 0.01, ns, not significant, log-rank (Mantel–Cox) test for significance. (E) Tumor growth curves for DTT-FDwt and PBS. Data are plotted as means ± SD. ns, not significant. (F,G) Design and purification of mutant peptide-based vaccines. (F) Schematic representation of DTT-SP4 and DTSP. SP represents residues 5–21 of KRAS containing the G12D mutation. DTSPwt represents wild-type DTSP lacking the KRAS G12D mutation. The texture box stands for the linker sequence (GG). The white box denotes the position of DTT from 88 to 95 replaced with SP. (G) Expression levels of DTT-SP4 (lane 1) and DTSP (lane 2).