Abstract
Two elements of neural information processing have primarily been proposed: firing rate and spike timing of neurons. In the case of synaptic plasticity, although spike-timing-dependent plasticity (STDP) depending on presynaptic and postsynaptic spike times had been considered the most common rule, recent studies have shown the inhibitory nature of the brain in vivo for precise spike timing, which is key to the STDP. Thus, the importance of the firing frequency in synaptic plasticity in vivo has been recognized again. However, little is understood about how the frequency-dependent synaptic plasticity (FDP) is regulated in vivo. Here, we focused on the presynaptic input pattern, the intracellular calcium decay time constants, and the background synaptic activity, which vary depending on neuron types and the anatomical and physiological environment in the brain. By analyzing a calcium-based model, we found that the synaptic weight differs depending on these factors characteristic in vivo, even if neurons receive the same input rate. This finding suggests the involvement of multifaceted factors other than input frequency in FDP and even neural coding in vivo.
Subject terms: Synaptic plasticity, Computational neuroscience
Introduction
Synaptic plasticity in neural networks is a substrate of learning and memory, which includes both positive and negative components, i.e., both long-lasting enhancements and declines in the weight of synaptic transmission (long-term potentiation (LTP) and long-term depression (LTD))1. Many experimental studies have suggested two plausible mechanisms for the induction of the synaptic plasticity2,3. The first is the frequency of spike trains, which has been studied in association with the Bienenstock, Cooper, and Munro (BCM) rule in classical research conducted approximately half a century ago4–6. LTP is induced by high-frequency firing in presynaptic neurons, which produces large increases in postsynaptic calcium concentration5–8. The low-frequency firing causes a modest increase in the calcium level, and thereby induces LTD9–11. The second is the precise timing of presynaptic and postsynaptic firing, which has been investigated as spike-time-dependent plasticity (STDP) in numerous experimental and theoretical studies from approximately 20 years ago12–15. LTP is induced by the presynaptic action potentials preceding postsynaptic spikes by no more than tens of milliseconds, whereas presynaptic firing that follows postsynaptic spikes produces LTD13,14,16–19. The idea that STDP plays a central role in synaptic plasticity had been becoming mainstream.
Recent studies have reported, however, that in some cases, the environment in vivo may not be suitable for precise spike timing, which is key to the STDP. Pre- and post-synaptic neurons in the primary visual cortex and extrastriate cortex of awaking animals fire so irregularly that the timing of presynaptic and postsynaptic firing varies20–22. Neurons and synapses in the cerebral cortex of rats receive a lot of background neuronal activity that is generated internally, which provides strong constraints on spike timing23–25. In these environments, the firing rate, rather than the spike timing, is likely to be important for the synaptic plasticity and neural coding. For example, it has been demonstrated experimentally that the cerebral cortex in which there is a high level of internal noise uses a rate code25, and it has been shown mathematically that synaptic changes are induced by variation of firing rate without any timing constraints20. The firing rate may also be an essential factor for the STDP. Recently, Madadi Asl et al. revealed that STDP model incorporating dendritic and axonal propagation delay can adequately explain the existence of recurrent connections between pairs of neurons in the cerebral cortex26. They found that the firing frequency plays an essential role in the formation of connectivity patterns in Two-Neuron Motif27. Moreover, firing variability, as well as the statistical properties of the spike frequency, seems essential for real-time information processing28.
Based on these reports, the role of firing frequency in various aspects of neural information processing has again come into the limelight. Furthermore, in vivo characteristic factors such as the variation of the firing pattern, the difference of intracellular parameters, and internal noise have also been suggested to be important for synaptic plasticity and neural coding20,28–32. However, how these factors are involved in the synaptic plasticity is poorly understood. In order to clarify this problem, we examined the role of the presynaptic input pattern, the intracellular calcium decay time constants, and the background synaptic activity in frequency-dependent synaptic plasticity (FDP) by analyzing a calcium-based model, which is one of the most compatible models with experimental results12,33.
Currently, it is widely accepted that the calcium concentration in the postsynapse determines whether LTP or LTD is induced34–37. A moderate elevation of intracellular calcium correlates with induction of LTD, whereas a larger increase correlates with LTP35,36. Only if glutamate is released by presynaptic activity and if the postsynaptic membrane is depolarized sufficiently, calcium ions enter the cell through channels controlled by NMDA receptors12. The depolarization of the postsynaptic membrane potential is due not only to excitatory postsynaptic potentials (EPSPs) generated by binding glutamate to the AMPA receptors but also to many kinds of background synaptic activities38–41. These experimental events were formulated by Shouval et al.33 as a calcium-based model (from now on, we call it “Shouval’s model”), which has been used in numerous studies.
In the present study, we investigated the FDP in vivo analytically and numerically using the Shouval’s model. First, to investigate the FDP in neurons with in vivo-specific firing pattern, we used three types of firing, which are widely observed in the brain, that is, constant-inter-spike intervals (ISI) inputs, Poisson inputs, and gamma inputs. Next, the calcium decay time constant of in vivo neurons varies from cell to cell. Previous reports suggested that pyramidal neurons in superficial layers possess faster calcium dynamics than those in deep layers. Here, ms in layer II to IV neurons, whereas ms in layer V to VI neurons42,43. To study the association of the calcium decay time constant with the FDP, we examined two kinds of neurons with time constants of 40 ms and 80 ms. Finally, neurons in vivo are constantly exposed to background synaptic activity38,41. The frequency and magnitude of this activity vary depending on the location of the synapse and the level of neuronal activity38,41. We, therefore, examined the correlation between the amplitude of background activity and the FDP. The findings in the present study may contribute to a detailed understanding of synaptic plasticity in in vivo brain.
Results
We used a model for the FDP based on the calcium control hypothesis of Shouval et al., assuming that the change of the synaptic weight is fully determined by the postsynaptic calcium level33,44. This model has been confirmed to integrate STDP observed in acute hippocampal slices within a single theoretical framework45. Among the few studies that have analytically solved this hypothesis, Yeung et al.46 calculated the mean values of the calcium transients evoked by a spiking neuron. In the present study, we analytically derived the intracellular calcium concentration and synaptic weight with respect to the input frequency focusing only on the long-term behavior of the intracellular calcium concentration and synaptic weight.
Postsynaptic calcium concentration as a function of the presynaptic stimulation frequency with fixed interstimulus intervals
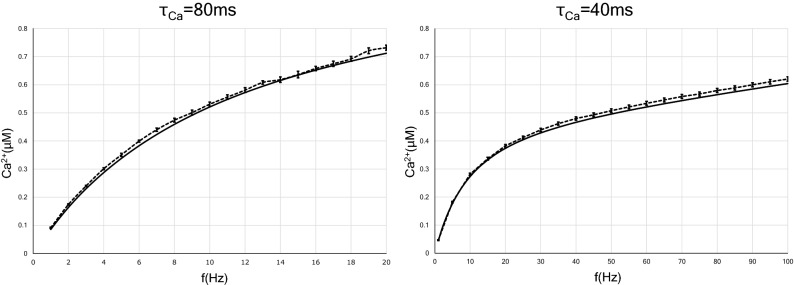
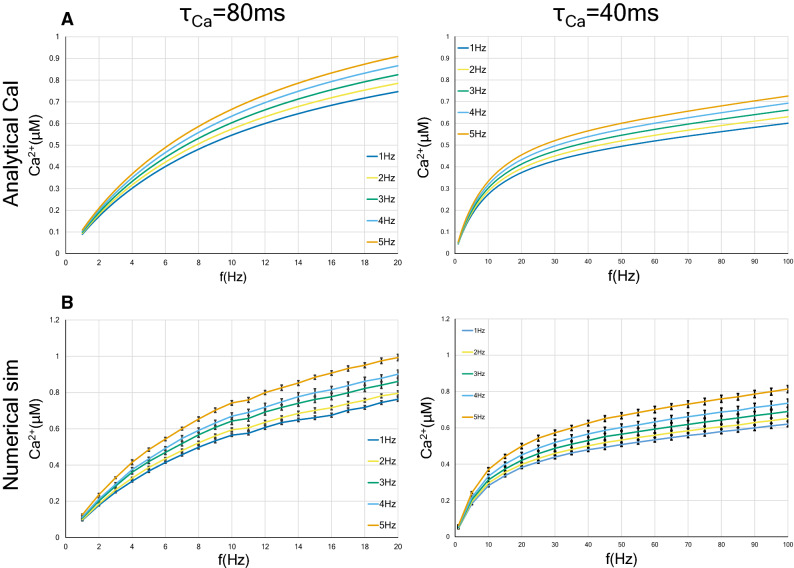
In order to investigate the dependence of the postsynaptic calcium concentration on the average presynaptic stimulation frequency of each input pattern, we first developed an analytical solution of the postsynaptic calcium concentration with constant-ISI inputs as a control. In Fig. 1, we plot the analytical solution of Ca in Eq. (42) as a function of the input frequency f. We also plot the simulation results obtained by solving Eqs. (9)–(18) numerically as a function of time and taking the time average of Ca for each frequency. The analytical solution for the long-term behavior of calcium level agrees very well with the numerical simulation results. We adopted ms for a long calcium decay time constant and ms as a short calcium decay time constant. The calcium concentration as a function of input frequency increases slower for ms than for ms. Equation (42) indicates that the calcium concentration at an arbitrary stimulation rate increases linearly for the calcium decay time constant .
Figure 1.
Presynaptic firing rate-induced elevation of intracellular calcium concentration in two types of neurons with different time constants of calcium decay. The analytic solution is indicated by solid lines, while the results of numerical calculation are indicated by dotted lines. The calcium level increases more slowly in neurons with the short calcium decay time constant (40 ms) than in neurons with the long decay time constant (80 ms). This is also understood from Eq. (42). Error bars indicate the standard error of the mean (SEM).
Approximate analytic solution of synaptic weight as a function of the input frequency with fixed interstimulus intervals
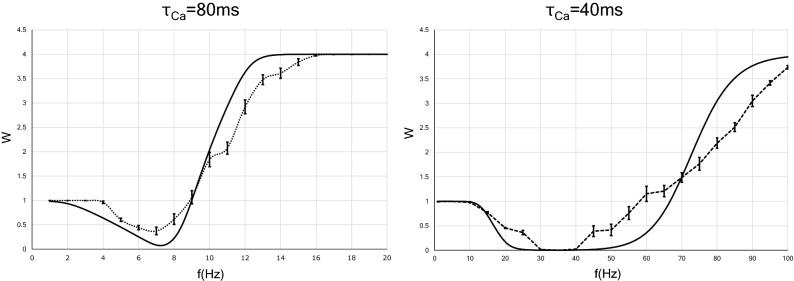
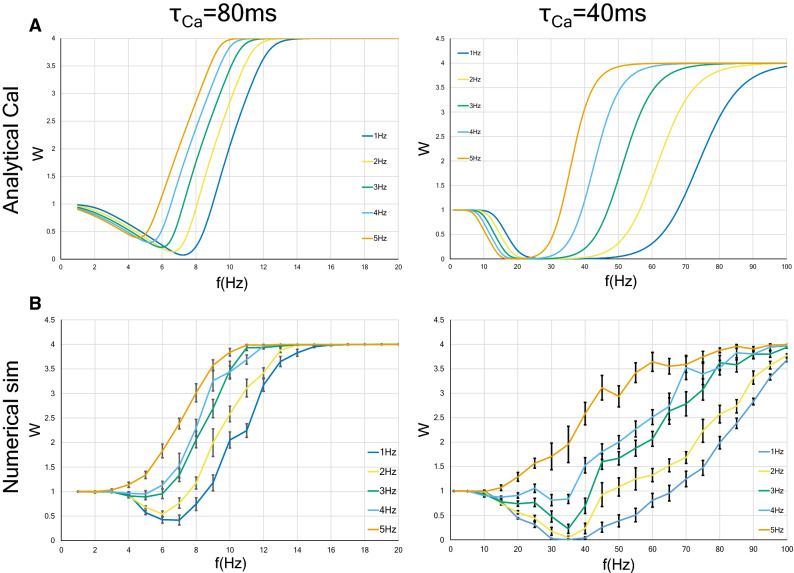
Figure 2 shows the curve obtained by performing the integration in Eq. (51). We also plot the results obtained by the numerical simulation, which agree qualitatively with the analytical results. These results suggest that the LTD/LTP threshold shifts to a lower frequency as the calcium decay time constant increases. Here, the LTD/LTP threshold is defined as the frequency at which the synaptic weight first returns to 1 after falling below 1 when the input frequency is increased from 0 Hz. This tendency can also be understood from Eq. (42) as follows. Equation (42) is written as , where F(f) is a monotonically increasing function of f, so that f can be formally expressed as
| 1 |
Figure 2.
Synaptic strength in two types of neurons that have different calcium decay time constants as a function of the constant presynaptic stimulation frequency. The x axis indicates the input frequency, and the y axis represents normalized synaptic weights that are obtained after several hundreds of presynaptic spikes. The analytic solutions are indicated by solid lines, whereas the solutions provided by numerical calculation are indicated by dotted lines. Error bars indicate the SEM.
Equations (11) and (49) indicate that when the synaptic strength is at the LTD/LTP threshold, the postsynaptic calcium level has a fixed value:
| 2 |
Substituting the numerical values of the parameters in Eq. (11) into Eq. (2), we obtained . Thus, the stimulation frequency when the synaptic weight reaches the LTD/LTP threshold is a monotonically increasing function of .
Postsynaptic calcium level and synaptic weight as functions of the average frequency of Poisson input
In several experimental studies on synaptic plasticity, the paradigms for inducing synaptic plasticity have consisted of constant-frequency stimulation trains, such as paired pulses or a tetanic stimulus. Neurons in vivo, however, are unlikely to experience such simple inputs. Rather, these neurons receive more complex input patterns in which ISIs are highly irregular47. The most representative stimulation patterns that are not constant-frequency stimulation trains are the Poisson process and the gamma process. In fact, spike sequences similar to these processes are sometimes observed in neurons of brain12,48–51. In this section, we discuss the results for the FDP of neurons with Poisson-distributed spike trains.
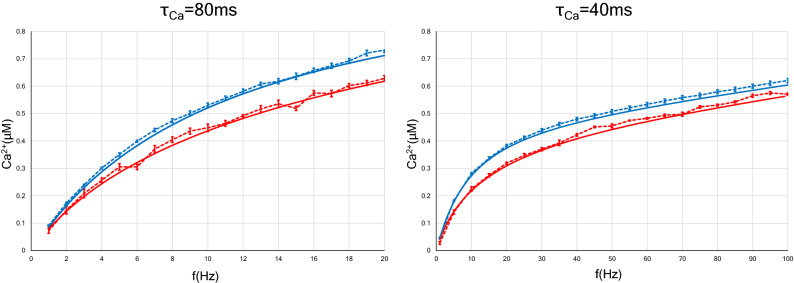
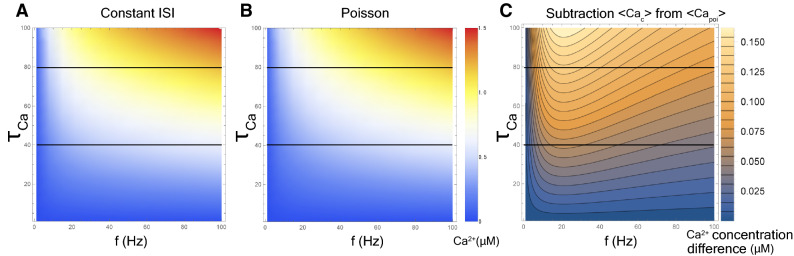
First, the calcium concentration at the postsynapse receiving Poisson input was calculated numerically and is plotted with red dotted lines in Fig. 3, in which the calcium concentration with constant-ISI input is also plotted with blue dotted lines for comparison. In the same manner, we examined two kinds of neurons with calcium decay time constants of 40 ms and 80 ms. The intracellular calcium concentration, regardless of the stimulation pattern, increases more gradually in the case of ms than in the case of ms. Besides, the calcium level with Poisson input rises more slowly than that with constant-ISI input, which is independent of the calcium decay time constant (Fig. 3, left and right panels).
Figure 3.
Relation between the postsynaptic calcium concentration and the average frequency of presynaptic constant-ISI (or Poisson) input. As in Fig. 1, two types of neurons with different calcium decay time constants were examined. The analytic solutions are shown with solid lines, whereas the results of numerical simulation are shown with dotted lines. The blue (or red) lines indicate the calcium concentration of the postsynapse with constant-ISI (or Poisson) input. In the case of Poisson input, the increase in calcium concentration with respect to the average frequency is slower than in the case of constant-ISI input. This result is independent of the calcium decay time constant. Error bars indicate the SEM.
Here we discuss how the calcium concentration increases with the input frequency. In Eqs. (42) and (44), the first-half part is a supra-linear function of the stimulation frequency f. This part describes the approximate expression of the voltage-dependence of the postsynaptic events given by H(V) in Eq. (14). The second-half parts in Eq. (42) and in Eq. (44) converge to 0 in the limit of infinite f. Thus, the competition between the two parts determines whether the calcium concentration in Eqs. (42) and (44) increases sublinearly or supralinearly. As shown in Fig. S1, in cases of both constant-ISI and Poisson input, the calcium concentration increases sublinearly in the frequency range between 0 and approximately 100 Hz, which is usually observed in the brain52. This is because the second-half parts of Eqs. (42) and (44) are dominant in this frequency range. The effect of the first-half part becomes stronger as the input frequency increases above about 100 Hz, so that the calcium concentration increases supralinearly.
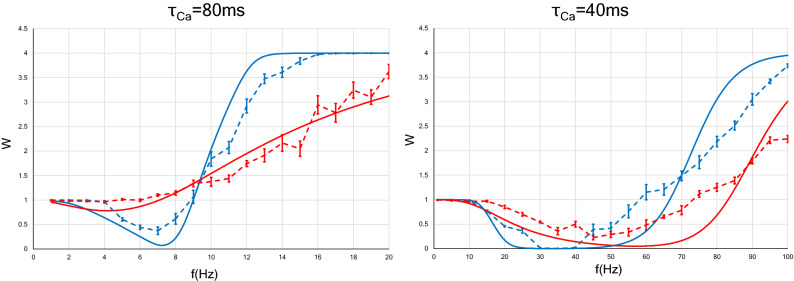
Next, we examined numerically the strength of a synapse receiving Poisson input. In Fig. 4, we define the LTD phase or LTP phase as the range of frequency indicating LTD or LTP. When the calcium decay time constant is 80 ms, interestingly, Poisson input makes the LTD phase disappear and the LTP phase is observed at any input frequency (see a dotted red line in Fig. 4, left panel), whereas in the case of constant-ISI stimulation, the LTD phase still exists at roughly between 3 and 9 Hz (see a dotted blue line in Fig. 4, left panel). When the calcium decay time constant is 40 ms, unlike in the case of ms, changing the stimulus pattern from constant-ISI input to Poisson input shifted the LTD/LTP threshold to the right (see dotted blue and red lines in Fig. 4, right panel). Since the firing rate observed in the brain is found to be at most approximately 112 Hz, we need only consider synaptic plasticity within 100 Hz52. This consideration leads to the conclusion that Poisson input to a neuron with ms expands the LTD phase and narrows the LTP phase. These results can be well reproduced by approximate analytical solutions [Eqs. (44) and (52)].
Figure 4.
Synaptic strength for two types of neurons ( ms and ms) as a function of the average rate of presynaptic stimulation. The x axis represents the input frequency, and the y axis represents normalized synaptic weights that are obtained after several hundreds of presynaptic spikes. The analytic solutions are shown with solid lines, whereas the results of numerical simulation are shown with dotted lines. Error bars indicate the SEM. The blue (or red) lines indicate the synaptic weights with constant-ISI (or Poisson) input. The synaptic weight with the Poisson input changes slowly compared to that with the constant-ISI input. As shown by the numerical simulation results, in neurons with ms, the Poisson input makes the LTD phase disappear, and only the LTP phase remains (a red dotted line in left panel). On the other hand, in neurons with ms, the LTD/LTP threshold moves to the right, and the LTD phase increases (red dotted line in right panel). These results are also qualitatively illustrated by analytical solutions (solid lines).
Analytical solutions for the calcium concentration with Poisson input [Eq. (44)] are plotted with red solid lines in Fig. 3, left and right panels. The solutions agree well with the numerical results(red dotted lines in Fig. 3) and indicate that Poisson stimulation gently increases the calcium concentration, as compared to constant-ISI input. This property does not depend on the calcium decay time constant (Fig. 5).
Figure 5.
Two-dimensional density plot of post-synaptic calcium concentration as a function of f and . We illustrate the calcium concentration with the constant-ISI input expressed in Eq. (42) (A) and with the Poisson input expressed in Eq. (44) (B). (C) Density plot of . The horizontal lines on each figure suggest corresponding values at ms and .
Next, we obtained an approximate expression for the relation between the synaptic weight and the average stimulation rate. By assuming that the synaptic weight W(t) converges to a stationary solution in the long-time scale [Eq. (49)], we obtain Eq. (52). Solid lines in Fig 4 show that the analytical expression agrees well with the results of the numerical simulation. Regardless of the value, the synaptic weight varies slowly by changing the stimulus pattern from constant-ISI input to Poisson input. This change in the stimulation pattern moves the LTD/LTP threshold to the left and narrows the LTD phase decrease for ms (see a solid red line in Fig. 4, left panel), whereas it has the opposite effect for ms (see a solid red line in Fig. 4, right panel).
Thus, the numerical and analytical studies indicate that the postsynaptic calcium concentration and synaptic strength receiving Poisson input behave differently from those receiving constant-ISI stimulation. At the same frequency, when ms, a synapse receiving Poisson input is more likely to be LTP than a synapse receiving constant-ISI input, and when ms, a synapse receiving Poisson input is more likely to be LTD. These findings suggest that the difference in input patterns (constant-ISI or Poisson input) and calcium decay time constant affects the output of FDP, i.e., LTD or LTP. In addition, this tendency to become LTP or LTD by changing the input pattern depends on the postsynaptic calcium decay time constant.
Postsynaptic calcium level and synaptic weight as a function of the average frequency of gamma process input
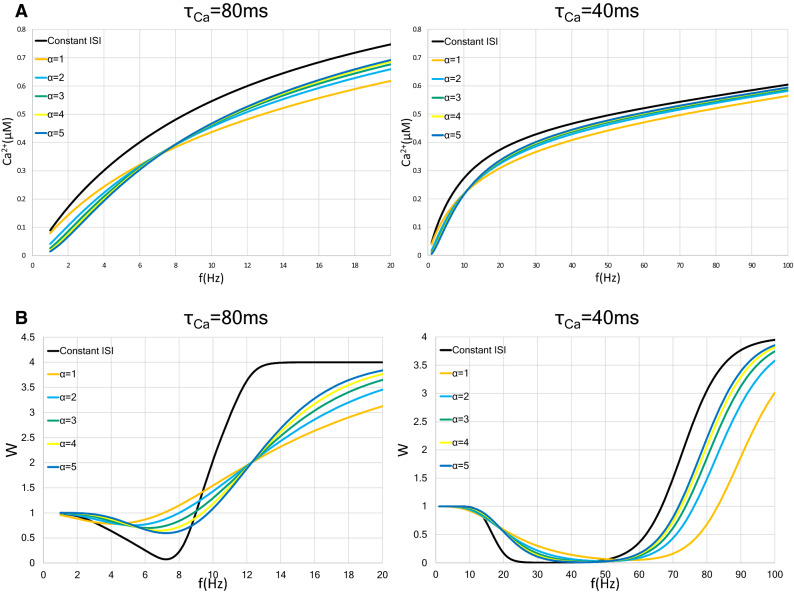
We studied the postsynaptic calcium concentration and synaptic load of neurons receiving gamma process inputs, which is one of the firing patterns observed in brain51,53. Since the analytic solutions are qualitatively consistent with the simulation results so far presented in the present paper, we discuss the plasticity of synapses receiving gamma process input by only the analytic solutions. The postsynaptic calcium concentration of neurons that receive gamma process input is expressed by Eq. (48), where is a shape parameter. The synaptic weight of the neurons receiving gamma process input is approximately expressed by Eq. (53) as a function of average input frequency.
This result for the calcium concentration is illustrated in Fig. 6A. As the shape parameter increases, the slope of the calcium concentration increases. The results for the synaptic weight are shown in Fig. 6B. When neurons with ms are stimulated by gamma process input, as the shape parameter increases, the LTD/LTP threshold shifts to a higher frequency and the minimum value of the synaptic weight becomes smaller (Fig. 6B, left). When ms, the LTD/LTP threshold shifts to a lower frequency as the shape parameter increases; on the other hand, the minimum value of the synaptic weight is approximately the same from to (Fig. 6B, right).
Figure 6.
Change in the postsynaptic calcium concentration and the weight in the synapse with gamma process input. We show two types of neurons with different time constants of calcium decay, ms and 40 ms. In each graph, the black, orange, light blue, blue green, yellow, and blue lines indicate constant-ISI input, shape parameter , , , , and , respectively. (A) Relationship between the postsynaptic intracellular calcium concentration and input frequency f in neurons stimulated with gamma process input. A graph of constant-ISI stimulation is shown as a control (black lines). The trace of the shape parameter matches the graph of the Poisson input. As the value of the shape parameter increases, the calcium level increases is faster. (B) Approximate relationship between synaptic weight and mean input frequency in neurons with constant-ISI and gamma process inputs. The LTD/LTP threshold moves to a higher frequency in the case of ms and the moves lower in the case of ms as the value of the shape parameter becomes large.
In summary, the postsynaptic calcium level with gamma process input increases slower than that with constant-ISI input, but increases faster than that with Poisson input. As the shape parameter increases, the increase in the calcium concentration becomes faster. The tendency to induce LTP or LTD by gamma process input depends on the shape parameter. These results suggest that the difference in input pattern as well as the shape parameter in gamma process input affects the synaptic weight.
Effect of increase in background synaptic activity receiving constant-ISI input
The postsynaptic terminals in neurons in vivo display intense background activity, which is characterized by fluctuations in the postsynaptic membrane potential. This background activity has at least three components: dendritic action potential, BPAPs, and voltage noise41,54. The voltage noise includes the stochastic properties of ion channels, the random release of neurotransmitter, and thermal noise. The distance from the soma or the differences in the cortical layer, in which neurons are located, affects the frequency and size of the amplitude of the background synaptic activity38–40.
In order to examine the FDP under various background synaptic activities, we first analytically and numerically calculated the dependence of the postsynaptic calcium concentration on the constant-ISI input under various frequencies of background Poisson input. The fluctuation of the membrane potential due to background synaptic activity is denoted by in Eq. (18). Since increases in proportion to the average frequency of the background synaptic activity , H(V) in Eq. (37) is approximately expressed as a bivariate quadratic function of f and . Thus, the postsynaptic calcium concentration is given as a function of f and as follows:
| 3 |
where , , , , , and . Figure 7A plots Eq. (3) using ms or ms. In both cases, the higher the average frequency of the background Poisson input is, the faster the rate of increase in the calcium concentration with synaptic input frequency becomes. As shown in Fig. 7B, qualitatively consistent results were obtained by numerical simulations.
Figure 7.
Postsynaptic calcium concentration in two types of neurons ( ms and ms) as a function of the frequency of presynaptic input and of the background input. The ISI of the presynaptic input is constant. The background Poisson input with a frequency in the range of 1 to 5 Hz was applied. The analytic solution is shown in (A), whereas the results of numerical simulation are shown in (B). Error bars indicate the SEM.
We next analytically and numerically calculated the relation between the synaptic weight and the input frequency under various background input rates. The approximate analytic solution is obtained as follows:
| 4 |
Here, is defined by Eq. (3) in Eq. (50). More explicitly, is given by
| 5 |
The analytical solution (4) is plotted in Fig. 8A, and the corresponding numerical solution is shown in Fig. 8B. Although the analytical solution captures the overall qualitative behavior of the numerical solution, one can see quantitative deviations. These deviations can be attributed to the fact as follow. First, as you can see from Eq. (16), the time fluctuation of the postsynaptic membrane potential V(t) elevates as the background input increases. Accordingly, the time dependence of H(V) in Eq. (14) or Eq. (20) cannot be ignored, which leads to the difficulty in establishing “Assumption 1” (see the “Methods” section). Second, as shown in the description of Eq. (42) in the “Methods” section, H(V) is approximated by a quadratic power series around the resting membrane potential. Therefore, when V moves away from the resting potential due to an increase in background activity, the approximation accuracy of H(V) deteriorates.
Figure 8.
Synaptic strength as a function of the frequency of presynaptic constant-ISI input and of the background Poisson input, under the background Poisson input with a frequency in the range of 1 to 5 Hz. Two types of neurons ( ms and ms) were examined. The analytic solution and the results of numerical calculation are shown in A and B, respectively. Error bars indicate the SEM.
Although two types of neurons with different calcium decay time constants were examined, the influence on the synaptic strengths by the increase of the background input level is qualitatively common to both types of neurons. In other words, the increase in the background input rate moves the LTD/LTP threshold to the left, decreases the LTD phase, and broadens the LTP phase.
Thus, upregulation of background synaptic activities leads to the enhancement of synaptic efficacy through the acceleration of the increasing rate of postsynaptic calcium concentration. These results suggest that the FDP output (LTP or LTD) varies depending on the magnitude of the applied background noise, even if the input frequency is the same.
We summarize the findings of the present study: (1) We obtained approximately analytical solutions of the intracellular calcium concentration and the synaptic weight as a function of the frequency of three kinds of input: constant-ISI, Poisson, and gamma process input. The latter two input patterns are often observed in vivo. (2) In all three input patterns, LTP occurs at a lower frequency as the calcium decay time constant increases. We used 80 ms as the longer calcium decay time constant () and 40 ms as the shorter calcium decay time constant. (3) The intracellular calcium level increases more slowly in neurons with Poisson input than in neurons with constant-ISI input. At the same stimulation frequency, a synapse with a long calcium decay time constant tends to be strengthened (LTP) by changing the stimulation pattern from constant-ISI input to Poisson input, while a synapse with a short calcium decay time constant weakened (LTD). (4) The calcium level with gamma process input increases faster than that with Poisson input but slower than that with constant-ISI input. Moreover, calcium level with gamma process also increases faster as the shape parameter grows. As the shape parameter increases, the LTD/LTP threshold moves to a higher frequency in ms neurons but moves to a lower frequency in ms neurons. The minimum value of the synaptic weight is smaller in ms neurons but is approximately constant in ms neurons as the shape parameter increases. (5) The increase of background synaptic activities induces the acceleration of the increase rate of the calcium level and the enhancement of synaptic weight.
Discussion
The Shouval’s model we studied is the most pioneering calcium-based model and is biophysically valid33,55. However, this model is quite complicated for analytical study. In the present study, focusing on only the long-term behavior of postsynaptic events, we derived approximate-analytic solutions from the Shouval’s model. Our results found from the analytic solutions indicate that the synaptic weight by FDP depends not only on input frequency but also on input pattern, shape parameter in gamma process input, calcium decay time constant, and background synaptic activity, which have been suggested to vary in vivo depending on the location, the internal state, and the external environment of the neuron41–43,51,53,54.
We now discuss the relevance of our study to some related prior works. Interestingly, conclusions similar to ours have been obtained from studies of some STDP models, which considered not intracellular mechanisms but only spike timing. The triplet-based model of STDP, which is much simpler than the Shouval’s model that was the basis of our research, explained the BCM rule and derived a similar conclusion to our Fig. 4, left panel, that is changing from regular to irregular spike patterns tends to evoke LTP20,56. Why are similar conclusions drawn from disparate models? Although all variables in equations of the triplet-based model are not identified as specific biophysical quantities, it is suggested that variables o1 and o2 in Eq. (2) in Pfister and Gerstner, 200656 may be related to the calcium current depending on the post-synaptic membrane potential, and the dynamics of the latest and second-to-latest spikes are considered. On the other hand, as can be seen from the Eqs. (23), (24) and (25), we separately calculated the contribution from the latest spike () and the contribution from all previous spikes ()), when analysing the calcium influx in the postsynapse depending on membrane potential. The triplet model seems to ignore the effect of the spikes other than the latest and second-to-latest ones. However, they decay exponentially quickly, and thus although these two studies differ in how far past spikes are calculated, they may be both good approximate representations of the post-synaptic events. Also, the results of the STDP model taking into account dendritic and axonal propagation delays, reported by Madadi Asl et al. do not contradict ours26,27. They showed that, in both two-neuron and network motifs, high-frequency firings promote bidirectional connections, indicating a large proportion of neurons with large synaptic weights. Low-frequency firings lead to unidirectional or decoupled connections, showing the decrease of the mean synaptic weight. These findings are similar to the BCM curve in the FDP (Fig. 2) and the induction of LTP by the increase in background postsynaptic inputs (Fig. 8B).
Graupner et al. proposed a calcium-based model that simplifies the Shouval’s model. They found that differences in plasticity outcomes are due to differences in parameters defining the calcium dynamics29. We have similar conclusions in this study; that is, LTP tends to occur even at a lower frequency as the calcium decay time constant increases (Fig. 2). Besides, applying firing patterns recorded in monkey area MT to the triplet-based model and the simplified calcium model, they found that synaptic plasticity can occur sufficiently with only the variation of firing rate without exact spike timing20. Furthermore, they investigated the effect of irregular input patterns on long-term plasticity with these models. They showed that irregular spike pairs tend to induce potentiation more than regular spike pairs. This conclusion is also similar to that obtained in this paper, that is, in ms neurons, changing from constant-ISI to Poisson input makes LTP easy to be induced (Fig. 4, left panel).
In addition to these conclusions, we obtained the following new findings. First, we found that Poisson stimulation evokes a lower calcium concentration than constant-ISI stimulation for the same input frequency (Figs. 3 and 5C). At a glance, this finding may appear counterintuitive, because, in the case of Poisson input, the probability of firing in the period between 0 ms and the time of average ISI is . It indicates that the proportion of firing with an ISI shorter than the average ISI is higher compared to that of firing with an ISI longer than the average ISI. However, the result we obtained is the opposite. To elucidate the reason for this, we obtained analytic solutions of the statistical average of the calcium current through NMDA receptor under constant-ISI and Poisson input as follows:
| 6 |
| 7 |
We also define the subtraction of Eq. (7) from Eq. (6) as follows:
| 8 |
In Fig. S2, we plot the analytical solution of in Eq. (8). Equations (8) and (13) indicate that, at any input frequency, the Poisson input leads to a smaller than the constant-ISI input, resulting in a smaller calcium concentration. For Poisson input, when firing with ISIs longer than the average, (EPSPs generated by binding glutamate to the AMPA receptors) and might decay much stronger than the case of firing with ISIs shorter than the average [cf. Eq. (17) and Eq. (20)].
Second, as can be seen from Eq. (1), where is a monotonically increasing function, we found that the LTD/LTP threshold under constant-ISI input can be written as a monotonically increasing function of . Moreover, we showed that the LTD/LTP threshold by changing from regular to irregular spikes shifts to lower frequency when is long (Fig. 4, left panel) and to higher frequency when is short (Fig. 4, right panel). It is necessary to examine whether the difference in the changes in the LTD/LTP threshold due to the calcium decay time constant is found in other calcium models such as the simplified calcium model and whether this phenomenon actually occurs in the brain.
For a long time, there has been a debate on the nature of neural coding, which is primarily founded on the generation, propagation, and processing of spikes57–59. The classical view of neural coding emphasizes the information carried by the rate at which neurons produce action potentials, whereas spike variability and background activity were ignored or treated as noise28,60,61. In experimental and theoretical studies of recent decades, arguing the importance of the spike timing rather than the firing rate in neural coding, the spike variability and background activity are also considered as noise activities12,25. However, the results of recent electrophysiological experiments on waking animals suggest that they are too large to be ignored for precise spike timing25,54, leading to a renewed awareness of the importance of the rate coding, which is less affected by individual spike variability and background noise20,21. Moreover, recent studies reveal the need for several simultaneous codes (multi-coding), including spike variability and fluctuation of membrane potential, as sources57,62–64. Hence, the multi-coding hypothesis for the neural coding problem may be supported by the results of the present study, suggesting that not only firing rate but also firing variability, the internal parameters of neurons, and the magnitude of background synaptic activity could be important for neural coding and synaptic plasticity28,57.
We found that the calcium decay time constant determines the plasticity outcome. In neurons with a long time constant, LTP is induced even by a small presynaptic rate (about 9 Hz), because the calcium concentration via the NMDA receptors increases faster in these neurons than in neurons with a short time constant (Figs. 1 and 2). In neurons with a short time constant, LTP is not induced until the stimulation frequency is large (over about 50 Hz). This difference due to calcium dynamics is more pronounced when the stimulation pattern is set to Poisson or gamma process input (Figs. 4 and 6).
The calcium decay time constant is closely related to the function of sodium-calcium exchangers (NCXs)65. Sodium-calcium exchangers, which are expressed highly in dendrites and dendritic spines in a variety of brain regions66, are controlled in activity by various intracellular and extracellular signaling molecules67 and are widely involved in many neural events from developmental processes to cognitive abilities68,69. Thus, the calcium decay time constant differs depending on anatomical and physiological characteristics. Indeed, previous reports suggest that the calcium decay time constant varies with the depth of the cerebral cortex and that nitric oxide stimulates the increase of the calcium decay time constant in a cGMP-dependent manner43,67,70. Our findings and those of previous studies suggest that, even with the same frequency, the synaptic plasticity induced thereby depends on the anatomical and physiological factors and that this difference becomes more prominent when the stimulation pattern is irregular.
Previous studies have demonstrated that applying an appropriate level of noise to the postsynapse results in the enhancement of the neural sensitivity and the improvement of signal detection in the central nervous system71,72. Consistent with these findings, our research indicates that increased synaptic noise is more likely to induce LTP, regardless of the calcium decay time constant. Recently, the dendritic action potential has been considered as one of the main components of synaptic noise. In the record of the dendritic membrane potential of freely behaving rats, dendrite spikes accompanied by large subthreshold membrane potential fluctuations occur with high rates greater than the BPAP evoked in the soma54. In addition, it has been shown in hippocampal synapses that even a single presynaptic burst induces LTP, provided dendritic action potentials are generated73. These findings and our results indicate that inputs from other than the presynapse, such as background synaptic activity, including the BPAP and the dendritic action potential, are largely involved in synaptic plasticity, especially the generation of LTP. We cannot, however, conclude from our results that even a single presynaptic input induces LTP. It is necessary to conduct research in which single-burst-induced LTP is substantiated experimentally. Therefore, a mathematical model that further improves the model used in the present study should be constructed.
Our study has the limitations as follows: (1) This study is a model study without experiments. By solving the Shouval’s model under various conditions analytically and numerically, we obtained conclusions that are similar to or novel over the previous studies. However, they are merely theoretical predictions, and whether they occur in the brain has to be verified by future experiments. (2) Since we analyzed only the long-term behavior of the Shouval’s model, the transient nature of synaptic plasticity in vivo was ignored. (3) Since our research uses the Shouval’s model, which considers only post-synaptic plasticity, we do not examine the presynaptic factors. However, in 2015, a new model of spike timing plasticity was proposed by Rui Ponte Costa et al. in which both pre- and post-synaptic plasticity were considered (referred to as “pre-post STDP model”)74. They used Poisson input as input pattern and showed that the pre-post STDP model induces higher SNR (signal-to-noise ratio) of a synaptic response than models considering only post-synaptic changes, and induces fast re-learning of stimuli experienced in the past. Therefore, it may be more appropriate to investigate, considering the pre- and post-synaptic plasticity, the influence on the synaptic weight by a difference in spike pattern, calcium dynamics, and background activity. It is necessary to clarify this point in the future. (4) We did not investigate the involvement of higher-order correlations. Gjorgjieva et al. analyzed the triplet-based model of STDP analytically and numerically55. They showed that, even when the input frequency is constant, the difference in spatial and spatiotemporal correlations determines the synaptic weight. It is a future task to study calcium dynamics and weight dynamics with highly correlated inputs to the calcium-based model. (5) We used only one model. It will be possible to deepen an understanding of synaptic plasticity by performing similar analysis as done in this paper on other mathematical models, STDP models and calcium models.
In conclusion, a problem regarding the FDP, namely, a firing rate abstraction, in which the temporal average of spikes is taken, is discussed, ignoring a large amount of extra information within the encoding window, such as the variation of firing pattern3,28,61. This loss of information contrasts the encoding of rapidly changing neuronal activity observed in the brain3,28. The present study showed theoretically that the output of synaptic plasticity in neurons receiving the same input frequency differs depending on the input pattern, the calcium decay time constant, and the background activity, which are related by neuron type and the anatomical and physiological condition in the brain. This finding suggests that information neglected in the view that only the firing rate induces the synaptic plasticity is also involved in the synaptic plasticity and neural coding. In the future, the ratio at which this information is related to synaptic plasticity and neural coding should be verified experimentally and theoretically.
Methods
Model
We used a model for the FDP based on the calcium control hypothesis of Shouval et al., assuming that the change of the synaptic weight is fully determined by the postsynaptic calcium level33,44.
The dynamics of the synaptic weight W(t) are governed by
| 9 |
where Ca(t) represents the intracellular calcium concentration, and and are functions of intracellular calcium concentration given by the following formulas:
| 10 |
| 11 |
where
| 12 |
and we used the following parameters: s, , , s, , and 33,44.
The dynamics of the intracellular calcium concentration are described as follows:
| 13 |
where is the calcium decay time constant. In order to investigate the relation between the calcium dynamics and the synaptic plasticity, we examined two kinds of neurons with time constants of 40 ms and 80 ms, which are known as representative values in pyramidal cells in the superficial cortex (layers II to IV) and the deep cortex (layers V to VI)42,43.
In Eq. (13), represents the calcium current via the NMDA receptor and is expressed as a function of time and postsynaptic potential as follows:
| 14 |
Here, is the Heaviside step function and we choose the parameters , , ms, and ms, and H(V) is given by
| 15 |
where we choose the parameters , , , and a reversal potential for calcium ions of 33. Since H(V) increases monotonically with the membrane potential V before reaching a plateau at , the higher the membrane potential the greater the calcium current through the NMDA receptor, , as long as .
The postsynaptic membrane potential is given as the sum of the resting membrane potential , which is set to mV, and the depolarization terms :
| 16 |
The depolarization terms in Eq. (16) include both EPSPs generated by binding glutamate to the AMPA receptors () and background contribution (), which describes the depolarization due to the factors other than EPSP. Here, is expressed as
| 17 |
where indicates the i-th presynaptic spike time, and the time constants are ms and ms33. Here, is composed of the summation of the dendritic action potentials, the back propagating action potentials (BPAPs), and the voltage noise applied to the postsynapse. The amplitude of the depolarization generated at the postsynaptic dendritic spine by the BPAPs varies, decreasing exponentially with the distance from the soma, at which it is about 100 mV relative to the synapse40,75. The duration of the depolarization by BPAPs also differs among cell types76. Moreover, the noise level at dendritic spines has been reported to be similar to that measured at the soma77. We took these previous studies into consideration in order to perform the numerical simulation and presumed that the spike trains by both BPAPs and voltage noise follow a homogeneous Poisson process. Thus, we simply expressed as follows:
| 18 |
where mV and is a Poisson process with a frequency that varies depending on the simulation conditions. (In all simulations except for those of Figs. 7 and 8, we used a Poisson process with a mean frequency of 1 Hz.)
Numerical simulations
In the present study, we performed numerical simulations as well as analytical calculations in order to investigate the FDP. We used Wolfram Mathematica software in all simulations, and determined the dependence of both the calcium concentration and the synaptic weight on the stimulation frequency as follows. First, we repeatedly solved Eqs. (9)–(18) numerically as a function of time for each frequency. The calcium concentration as a function of time obtained by this calculation is similar to the results of a previous paper46. Next, after a period of ms, which is necessary for the system to reach a steady state, the average of the calcium level or the synaptic efficacy between ms to ms was calculated. When simulating with Poisson inputs, we performed the above calculations for at least three input patterns by changing the random seed, and took the average. The quantitative data are expressed as the mean of ten independent experiments plus/minus the standard error of the mean (SEM).
Derivation of the analytic solutions of the postsynaptic calcium concentration as functions of the average frequency of constant-ISI, Poisson, and gamma process inputs
By integrating Eq. (13), we can formally express the solution for Ca(t) as
| 19 |
Considering that the ion current through NMDAR () is reset to zero each time presynaptic input is applied, Eq. (14) is rewritten as follows for the interval between the presynaptic inputs , where is the time for k-th presynaptic input ():
| 20 |
Now, we make the following assumptions.
Assumption 1
The time dependence of H(V) can be neglected because it varies slowly in time compared to the other terms in Eq. (20)
Assumption 2
The spike interval fluctuates stochastically. If we define the average spike interval as , is written as follows:
| 21 |
Then,
| 22 |
Inserting Eq. (20) into Eq. (19) with Assumption 1, we obtain
| 23 |
where we have separated the contributions from the N-th presynaptic input, and from the contributions from the first presynaptic inputs, and :
| 24 |
and
| 25 |
Furthermore, we define as
| 26 |
where and are defined as follows:
| 27 |
We write , where represents the time interval between the last spike time () and the time to measure the calcium concentration (t). Substituting the formula into Eq. (26), we obtain
| 28 |
In the case of , we have
| 29 |
In the case of , we have
| 30 |
Since we are interested in the long-term behavior of the calcium concentration and synaptic weights, but not in the fluctuations caused by each spike, we take the statistical average over one cycle. Let in Assumption 2 obey the probability density function . Then the statistical averages of and can be written as
| 31 |
Hence, the statistical average of Eq. (29) is given as
| 32 |
Summing from to , the statistical average of Eq. (24) is obtained as
| 33 |
In order to obtain the long-term behavior of , we take the limit . Since , and thus as , we obtain
| 34 |
Similarly, using in Eq. (25) and taking the statistical average, we obtain (in the limit )
| 35 |
Using Eqs. (34) and (35), we obtain the statistical average of the postsynaptic calcium concentration as
| 36 |
Furthermore, the statistical average of this equation with respect to the observation time is given by
| 37 |
where
| 38 |
Calcium concentration of constant-ISI input
First, we calculate , , , and for the constant-ISI input, which are denoted as , , , and , respectively. In this case, the probability density function is given by . Using this function in Eq. (31), we obtain
| 39 |
Since it is assumed that the sampling time follows a uniform distribution, and are expressed as follows:
| 40 |
Using Eqs. (39) and (40), we obtain the statistical average of the postsynaptic calcium concentration as a function of the spike interval as follows:
| 41 |
Note that H(V) is a slowly changing and monotonically increasing function of the membrane potential in the vicinity of the resting membrane potential (− 65 mV), and the duration of depolarization by EPSP is approximately 50 to 100 ms at most. Therefore, the increase in the average membrane potential remains at approximately 5.4 mV, even in the case of the highest frequency, e.g., 100 Hz. The average membrane potential, moreover, increases linearly with the stimulation frequency. Thus, is approximately expressed as a quadric function of . With this approximation, we obtain the following expression:
| 42 |
Here, , , and . These values are determined by finding the relation between the input frequency and the time average of V(t) in Eq. (16) and by substituting the obtained values into the quadratic approximation of H(V).
Calcium concentration of Poisson input
The time interval of the spike sequence according to the Poisson process follows an exponential distribution, the probability density function of which is given by . Then, we can calculate and for the Poisson input as
| 43 |
Since the spike interval fluctuates stochastically in the Poisson input, the observation time is considered to fluctuate with the same statistics. Then, and in the Poisson input, written as and , are equal to and , respectively. Substituting , , , and , we obtain the statistical average of the postsynaptic calcium concentration receiving Poisson input as a function of the average frequency as follows:
| 44 |
Calcium concentration of gamma process input
The time interval of the spike sequence according to the gamma process follows a gamma distribution, the general formula for the probability density function of which is given as
| 45 |
where is the shape parameter, and is the gamma function, which is given by
| 46 |
Since, as in the Poisson input, the spike interval and the sampling time fluctuate with the same statistics, and in Eq. (36). Thus, we obtain
| 47 |
Noting that the average spike interval of the gamma distribution input is , we can express the statistical average of the postsynaptic calcium concentration with gamma process input as follows:
| 48 |
Derivation of the approximate analytic solutions for the synaptic weight as functions of the average frequency of constant-ISI, Poisson, and gamma process inputs
According to the calcium control hypothesis reported by Shouval et al., the time derivative of the synaptic efficacy W is expressed as a function of intracellular calcium concentration as indicated in Eqs. (9)–(11)33. Equation (9) indicates that the synaptic strength approaches an asymptotic value with time constant . The functional form of in Eq. (11) is based qualitatively on the notion that a moderate rise in calcium leads to a decrease in the synaptic weight, whereas a large rise leads to an increase in the synaptic weight. This notion is closely related to the BCM theory, which states that weak synaptic input activity results in a decrease in synaptic strength, whereas strong input leads to an increase in synaptic weight4,78.
Although it is difficult to find the exact relation between the synaptic weight W and the stimulation rate f analytically, we can obtain an approximate relation by assuming that W(t) converges to a stationary solution in the macroscopic time scale, i.e.,
| 49 |
In order to calculate , we express the postsynaptic calcium concentration as
| 50 |
where , , and are defined in Eq. (31). By substituting Eq. (50) into the expression for in Eq. (11) and calculating the statistical average with respect to x and , we obtain an approximate analytical solution for the synaptic weight as a function of the average input frequency.
In the case of the constant-ISI input, the time interval of the spike sequence obeys the probability density function . Moreover, the time interval from the last spike to the sampling time obeys a uniform distribution. Thus, we obtain the statistical average of the synaptic weight as a function of input frequency f as follows:
| 51 |
In the cases of the Poisson input and gamma process input, the spike interval as well as the time interval between the last spike and the observation time obey exponential and gamma distributions, respectively. Thus, the statistical average of the synaptic weight as a function of input frequency f in these inputs are calculated as follows:
| 52 |
| 53 |
Supplementary information
Acknowledgements
This work was supported by JSPS KAKENHI Grant Numbers JP18K10858, JP18K10999.
Author contributions
K.H. conceived of the presented idea. K.H. developed the theory. K.H. and O.A. designed the model and the computational framework. K.H. and T.N. performed the analytical calculation. K.H. and O.Y. performed the numerical simulation. K.H., T.K., Y.Y., S.I. and T.N. analysed the data. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-70876-4.
References
- 1.Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu. Rev. Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 2.Sjostrom PJ, Turrigiano GG, Nelson SB. Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron. 2001;32:1149–64. doi: 10.1016/S0896-6273(01)00542-6. [DOI] [PubMed] [Google Scholar]
- 3.Weissenberger F, Gauy MM, Lengler J, Meier F, Steger A. Voltage dependence of synaptic plasticity is essential for rate based learning with short stimuli. Sci. Rep. 2018;8:4609. doi: 10.1038/s41598-018-22781-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J. Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973;232:331–56. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bliss TV, Gardner-Medwin AR. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J. Physiol. 1973;232:357–74. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 8.Kirkwood A, Dudek SM, Gold JT, Aizenman CD, Bear MF. Common forms of synaptic plasticity in the hippocampus and neocortex in vitro. Science. 1993;260:1518–21. doi: 10.1126/science.8502997. [DOI] [PubMed] [Google Scholar]
- 9.Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-d-aspartate receptor blockade. Proc. Natl. Acad. Sci. USA. 1992;89:4363–7. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–75. doi: 10.1016/0896-6273(92)90248-C. [DOI] [PubMed] [Google Scholar]
- 11.Artola A, Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci. 1993;16:480–7. doi: 10.1016/0166-2236(93)90081-V. [DOI] [PubMed] [Google Scholar]
- 12.Gerstner W, Kistler WM. Spiking Neuron Models : Single Neurons, Populations, Plasticity. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- 13.Song S, Miller KD, Abbott LF. Competitive hebbian learning through spike-timing-dependent synaptic plasticity. Nat. Neurosci. 2000;3:919–26. doi: 10.1038/78829. [DOI] [PubMed] [Google Scholar]
- 14.Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: Dependence on spike timing, synaptic strength, and postsynaptic cell type. J. Neurosci. 1998;18:10464–72. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerstner W, Kempter R, van Hemmen JL, Wagner H. A neuronal learning rule for sub-millisecond temporal coding. Nature. 1996;383:76–81. doi: 10.1038/383076a0. [DOI] [PubMed] [Google Scholar]
- 16.Markram H, Lubke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–5. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- 17.Debanne D, Gahwiler BH, Thompson SM. Long-term synaptic plasticity between pairs of individual CA3 pyramidal cells in rat hippocampal slice cultures. J. Physiol. 1998;507(Pt 1):237–47. doi: 10.1111/j.1469-7793.1998.237bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldman DE. Timing-based LTP and LTD at vertical inputs to layer II/III pyramidal cells in rat barrel cortex. Neuron. 2000;27:45–56. doi: 10.1016/S0896-6273(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 19.Zhang LI, Tao HW, Holt CE, Harris WA, Poo M. A critical window for cooperation and competition among developing retinotectal synapses. Nature. 1998;395:37–44. doi: 10.1038/25665. [DOI] [PubMed] [Google Scholar]
- 20.Graupner M, Wallisch P, Ostojic S. Natural firing patterns imply low sensitivity of synaptic plasticity to spike timing compared with firing rate. J. Neurosci. 2016;36:11238–11258. doi: 10.1523/JNEUROSCI.0104-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Softky WR, Koch C. The highly irregular firing of cortical cells is inconsistent with temporal integration of random EPSPs. J. Neurosci. 1993;13:334–50. doi: 10.1523/JNEUROSCI.13-01-00334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knierim JJ, van Essen DC. Neuronal responses to static texture patterns in area v1 of the alert macaque monkey. J. Neurophysiol. 1992;67:961–80. doi: 10.1152/jn.1992.67.4.961. [DOI] [PubMed] [Google Scholar]
- 23.Chance F, Abbott LF. Simulating in Vivo Background Activity in a Slice With The Dynamic Clamp. Berlin: Springer; 2009. pp. 73–87. [Google Scholar]
- 24.Jacobson GA, et al. Subthreshold voltage noise of rat neocortical pyramidal neurones. J. Physiol. 2005;564:145–60. doi: 10.1113/jphysiol.2004.080903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.London M, Roth A, Beeren L, Hausser M, Latham PE. Sensitivity to perturbations in vivo implies high noise and suggests rate coding in cortex. Nature. 2010;466:123–7. doi: 10.1038/nature09086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madadi Asl M, Valizadeh A, Tass PA. Dendritic and axonal propagation delays determine emergent structures of neuronal networks with plastic synapses. Sci. Rep. 2017;7:39682. doi: 10.1038/srep39682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madadi Asl M, Valizadeh A, Tass PA. Delay-induced multistability and loop formation in neuronal networks with spike-timing-dependent plasticity. Sci. Rep. 2018;8:12068. doi: 10.1038/s41598-018-30565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M, Tsien JZ. Neural code-neural self-information theory on how cell-assembly code rises from spike time and neuronal variability. Front. Cell Neurosci. 2017;11:236. doi: 10.3389/fncel.2017.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graupner M, Brunel N. Calcium-based plasticity model explains sensitivity of synaptic changes to spike pattern, rate, and dendritic location. Proc. Natl. Acad. Sci. USA. 2012;109:3991–6. doi: 10.1073/pnas.1109359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sjostrom PJ, Hausser M. A cooperative switch determines the sign of synaptic plasticity in distal dendrites of neocortical pyramidal neurons. Neuron. 2006;51:227–38. doi: 10.1016/j.neuron.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 31.Nobukawa S, Nishimura H. Enhancement of spike-timing-dependent plasticity in spiking neural systems with noise. Int. J. Neural Syst. 2016;26:1550040. doi: 10.1142/S0129065715500409. [DOI] [PubMed] [Google Scholar]
- 32.Yasuda H, et al. Novel class of neural stochastic resonance and error-free information transfer. Phys. Rev. Lett. 2008;100:118103. doi: 10.1103/PhysRevLett.100.118103. [DOI] [PubMed] [Google Scholar]
- 33.Shouval HZ, Bear MF, Cooper LN. A unified model of nmda receptor-dependent bidirectional synaptic plasticity. Proc. Natl. Acad. Sci. USA. 2002;99:10831–6. doi: 10.1073/pnas.152343099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cummings JA, Mulkey RM, Nicoll RA, Malenka RC. Ca signaling requirements for long-term depression in the hippocampus. Neuron. 1996;16:825–33. doi: 10.1016/S0896-6273(00)80102-6. [DOI] [PubMed] [Google Scholar]
- 35.Cormier RJ, Greenwood AC, Connor JA. Bidirectional synaptic plasticity correlated with the magnitude of dendritic calcium transients above a threshold. J. Neurophysiol. 2001;85:399–406. doi: 10.1152/jn.2001.85.1.399. [DOI] [PubMed] [Google Scholar]
- 36.Cho K, Aggleton JP, Brown MW, Bashir ZI. An experimental test of the role of postsynaptic calcium levels in determining synaptic strength using perirhinal cortex of rat. J. Physiol. 2001;532:459–66. doi: 10.1111/j.1469-7793.2001.0459f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang SN, Tang YG, Zucker RS. Selective induction of LTP and LTD by postsynaptic [Ca]i elevation. J. Neurophysiol. 1999;81:781–7. doi: 10.1152/jn.1999.81.2.781. [DOI] [PubMed] [Google Scholar]
- 38.Jones RS, Woodhall GL. Background synaptic activity in rat entorhinal cortical neurones: Differential control of transmitter release by presynaptic receptors. J. Physiol. 2005;562:107–20. doi: 10.1113/jphysiol.2004.076133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rapp M, Yarom Y, Segev I. Modeling back propagating action potential in weakly excitable dendrites of neocortical pyramidal cells. Proc. Natl. Acad. Sci. USA. 1996;93:11985–90. doi: 10.1073/pnas.93.21.11985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bereshpolova Y, Amitai Y, Gusev AG, Stoelzel CR, Swadlow HA. Dendritic backpropagation and the state of the awake neocortex. J. Neurosci. 2007;27:9392–9. doi: 10.1523/JNEUROSCI.2218-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faisal AA, Selen LP, Wolpert DM. Noise in the nervous system. Nat. Rev. Neurosci. 2008;9:292–303. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed B, Anderson JC, Douglas RJ, Martin KA, Whitteridge D. Estimates of the net excitatory currents evoked by visual stimulation of identified neurons in cat visual cortex. Cereb. Cortex. 1998;8:462–76. doi: 10.1093/cercor/8.5.462. [DOI] [PubMed] [Google Scholar]
- 43.Liu YH, Wang XJ. Spike-frequency adaptation of a generalized leaky integrate-and-fire model neuron. J. Comput. Neurosci. 2001;10:25–45. doi: 10.1023/A:1008916026143. [DOI] [PubMed] [Google Scholar]
- 44.Shouval HZ, Kalantzis G. Stochastic properties of synaptic transmission affect the shape of spike time-dependent plasticity curves. J. Neurophysiol. 2005;93:1069–73. doi: 10.1152/jn.00504.2004. [DOI] [PubMed] [Google Scholar]
- 45.Bush D, Jin Y. Calcium control of triphasic hippocampal STDP. J. Comput. Neurosci. 2012;33:495–514. doi: 10.1007/s10827-012-0397-5. [DOI] [PubMed] [Google Scholar]
- 46.Yeung LC, Castellani GC, Shouval HZ. Analysis of the intraspinal calcium dynamics and its implications for the plasticity of spiking neurons. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2004;69:011907. doi: 10.1103/PhysRevE.69.011907. [DOI] [PubMed] [Google Scholar]
- 47.Speed HE, Dobrunz LE. Developmental decrease in short-term facilitation at schaffer collateral synapses in hippocampus is mGluR1 sensitive. J. Neurophysiol. 2008;99:799–813. doi: 10.1152/jn.00625.2007. [DOI] [PubMed] [Google Scholar]
- 48.Brunel N. Dynamics of sparsely connected networks of excitatory and inhibitory spiking neurons. J. Comput. Neurosci. 2000;8:183–208. doi: 10.1023/A:1008925309027. [DOI] [PubMed] [Google Scholar]
- 49.Deger M, Helias M, Boucsein C, Rotter S. Statistical properties of superimposed stationary spike trains. J. Comput. Neurosci. 2012;32:443–63. doi: 10.1007/s10827-011-0362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maimon G, Assad JA. Beyond poisson: Increased spike-time regularity across primate parietal cortex. Neuron. 2009;62:426–40. doi: 10.1016/j.neuron.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baker SN, Lemon RN. Precise spatiotemporal repeating patterns in monkey primary and supplementary motor areas occur at chance levels. J. Neurophysiol. 2000;84:1770–80. doi: 10.1152/jn.2000.84.4.1770. [DOI] [PubMed] [Google Scholar]
- 52.Burton SD, Urban NN. Rapid feedforward inhibition and asynchronous excitation regulate granule cell activity in the mammalian main olfactory bulb. J. Neurosci. 2015;35:14103–22. doi: 10.1523/JNEUROSCI.0746-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li, M. et al. Spike-timing pattern operates as gamma-distribution across cell types, regions and animal species and is essential for naturally-occurring cognitive states. Biorxiv 145813 (2018).
- 54.Moore JJ, et al. Dynamics of cortical dendritic membrane potential and spikes in freely behaving rats. Science. 2017 doi: 10.1126/science.aaj1497. [DOI] [PubMed] [Google Scholar]
- 55.Gjorgjieva J, Clopath C, Audet J, Pfister JP. A triplet spike-timing-dependent plasticity model generalizes the Bienenstock–Cooper–Munro rule to higher-order spatiotemporal correlations. Proc. Natl. Acad. Sci. USA. 2011;108:19383–8. doi: 10.1073/pnas.1105933108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pfister JP, Gerstner W. Triplets of spikes in a model of spike timing-dependent plasticity. J. Neurosci. 2006;26:9673–82. doi: 10.1523/JNEUROSCI.1425-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carrillo-Medina JL, Latorre R. Implementing signature neural networks with spiking neurons. Front. Comput. Neurosci. 2016;10:132. doi: 10.3389/fncom.2016.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bialek W, Rieke F, de Ruyter van Steveninck RR, Warland D. Reading a neural code. Science. 1991;252:1854–7. doi: 10.1126/science.2063199. [DOI] [PubMed] [Google Scholar]
- 59.Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 5. New York: Elsevier Science Publishing Co., Inc.; 2013. [Google Scholar]
- 60.Rao RPN, Olshausen BA, Lewicki MS. Probabilistic models of the brain: Perception and neural function. In: Rao RPN, Olshausen BA, Lewicki MS, editors. Neural Information Processing Series. Cambridge: MIT Press; 2002. [Google Scholar]
- 61.Zhao C, et al. Spike-time-dependent encoding for neuromorphic processors. J. Emerg. Technol. Comput. Syst. 2015;12:1–21. doi: 10.1145/2738040. [DOI] [Google Scholar]
- 62.Panzeri S, Brunel N, Logothetis NK, Kayser C. Sensory neural codes using multiplexed temporal scales. Trends Neurosci. 2010;33:111–20. doi: 10.1016/j.tins.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 63.Latorre R, Rodriguez FB, Varona P. Neural signatures: Multiple coding in spiking-bursting cells. Biol. Cybern. 2006;95:169–83. doi: 10.1007/s00422-006-0077-5. [DOI] [PubMed] [Google Scholar]
- 64.Kayser C, Montemurro MA, Logothetis NK, Panzeri S. Spike-phase coding boosts and stabilizes information carried by spatial and temporal spike patterns. Neuron. 2009;61:597–608. doi: 10.1016/j.neuron.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 65.Hu E, et al. A glutamatergic spine model to enable multi-scale modeling of nonlinear calcium dynamics. Front. Comput. Neurosci. 2018;12:58. doi: 10.3389/fncom.2018.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Minelli A, et al. Cellular and subcellular localization of Na–Ca exchanger protein isoforms, NCX1, NCX2, and NCX3 in cerebral cortex and hippocampus of adult rat. Cell Calcium. 2007;41:221–34. doi: 10.1016/j.ceca.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 67.Jeon D, et al. Enhanced learning and memory in mice lacking Na/Ca exchanger 2. Neuron. 2003;38:965–76. doi: 10.1016/S0896-6273(03)00334-9. [DOI] [PubMed] [Google Scholar]
- 68.Secondo A, et al. Involvement of the Na/Ca exchanger isoform 1 (NCX1) in neuronal growth factor (NGF)-induced neuronal differentiation through Ca-dependent AKT phosphorylation. J. Biol. Chem. 2015;290:1319–31. doi: 10.1074/jbc.M114.555516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moriguchi S, et al. Reduced expression of Na/Ca exchangers is associated with cognitive deficits seen in Alzheimer’s disease model mice. Neuropharmacology. 2018;131:291–303. doi: 10.1016/j.neuropharm.2017.12.037. [DOI] [PubMed] [Google Scholar]
- 70.Asano S, et al. Nitroprusside and cyclic GMP stimulate Na-Ca exchange activity in neuronal preparations and cultured rat astrocytes. J. Neurochem. 1995;64:2437–41. doi: 10.1046/j.1471-4159.1995.64062437.x. [DOI] [PubMed] [Google Scholar]
- 71.Destexhe A, Rudolph M, Pare D. The high-conductance state of neocortical neurons in vivo. Nat. Rev. Neurosci. 2003;4:739–51. doi: 10.1038/nrn1198. [DOI] [PubMed] [Google Scholar]
- 72.Stacey WC, Durand DM. Synaptic noise improves detection of subthreshold signals in hippocampal CA1 neurons. J. Neurophysiol. 2001;86:1104–12. doi: 10.1152/jn.2001.86.3.1104. [DOI] [PubMed] [Google Scholar]
- 73.Remy S, Spruston N. Dendritic spikes induce single-burst long-term potentiation. Proc. Natl. Acad. Sci. USA. 2007;104:17192–7. doi: 10.1073/pnas.0707919104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Costa RP, Froemke RC, Sjostrom PJ, van Rossum MC. Unified pre- and postsynaptic long-term plasticity enables reliable and flexible learning. Elife. 2015 doi: 10.7554/eLife.09457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stuart GJ, Hausser M. Dendritic coincidence detection of EPSPs and action potentials. Nat. Neurosci. 2001;4:63–71. doi: 10.1038/82910. [DOI] [PubMed] [Google Scholar]
- 76.Zheng Y, Schwabe L. Shaping synaptic learning by the duration of postsynaptic action potential in a new STDP model. PLoS ONE. 2014;9:e88592. doi: 10.1371/journal.pone.0088592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yaron-Jakoubovitch A, Jacobson GA, Koch C, Segev I, Yarom Y. A paradoxical isopotentiality: A spatially uniform noise spectrum in neocortical pyramidal cells. Front. Cell Neurosci. 2008;2:3. doi: 10.3389/neuro.03.003.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Izhikevich EM, Desai NS. Relating stdp to bcm. Neural Comput. 2003;15:1511–23. doi: 10.1162/089976603321891783. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.