Fig. 6.
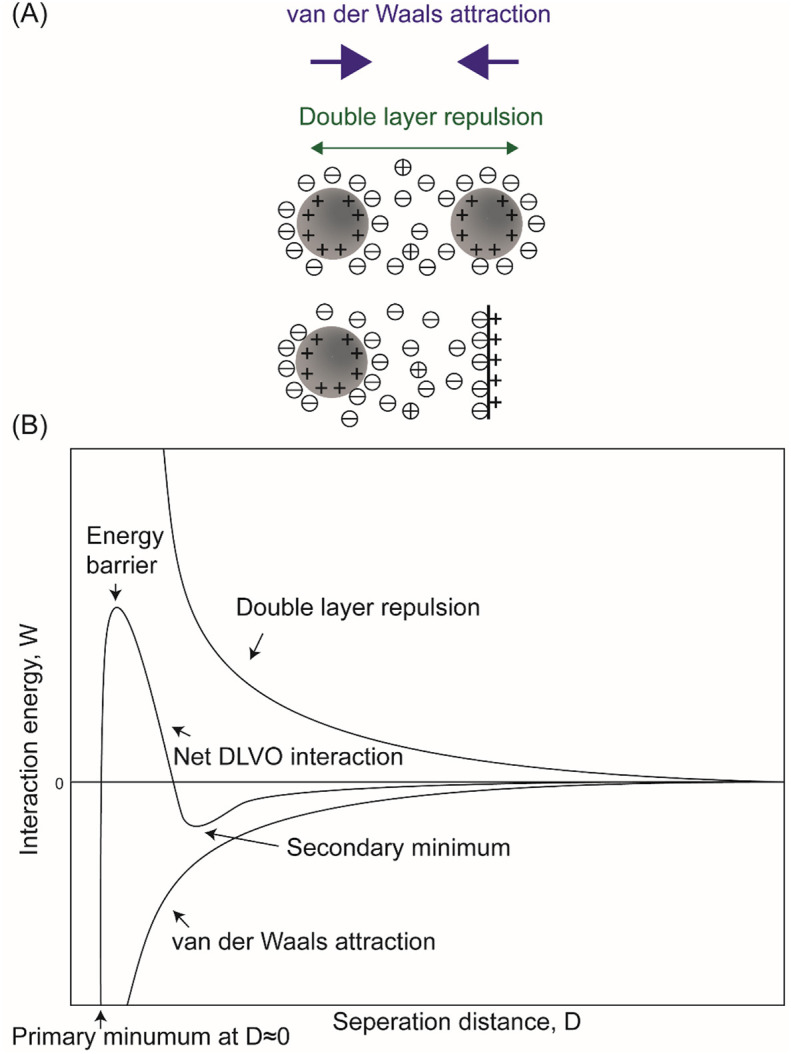
(A) Schematics of DLVO interactions between positively charged particle-particle and particle-surface systems in an aqueous solution. For surfaces carrying similar charges in the aqueous media, their DLVO interactions combine the effect of electrical double layer repulsion and van der Waals attraction. (B) Schematic plots of DLVO interaction energy vs. separation distance between similarly charged surfaces or colloidal particles dispersed in aqueous solutions. The double layer force exists as relatively long-rang repulsion. The net DLVO interaction has a high peak known as the energy barrier at high charge density and low electrolyte concentration. In concentrated electrolyte solution, a secondary minimum would appear at some critical separation, while the primary minimum is present when the interacting surfaces are in contact. When the surface charge densities are high in solutions with dilute electrolytes, the surfaces repel each other as the double layer force dominates. When the charge densities are below a certain value or the electrolyte concentration is higher than the critical coagulation concentration, the energy barrier falls below 0, giving rise to rapid coagulation [188].
