Table 5.
Results of meta-analysis and Grading of Recommendations Assessment, Development and Evaluation assessments for objective secondary outcomes.
| Outcomes | Trials included | Sample size | SMDa (95% CI) | P value | I 2 | Egger test | Quality of evidence (GRADEb) | |
|
|
|
|
|
|
|
t value | P value |
|
| FBGc | 6 | 416 | −0.29 (−0.49 to −0.10) | .004 | 2 | 2.27 | .09 |
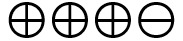 Moderated Moderated
|
| Waist circumference | 4 | 433 | −0.23 (−0.43 to −0.04) | .02 | 0 | 0.60 | .61 |
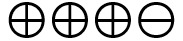 Moderated Moderated
|
| Body weight | 9 | 682 | −0.09 (−0.24 to 0.07) | .97 | 0 | 0.02 | .98 |
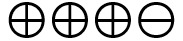 Moderated Moderated
|
| BMI | 6 | 575 | −0.06 (−0.23 to 0.12) | .53 | 12 | 3.36 | .03 |
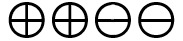 Lowd,e Lowd,e
|
| Total cholesterol | 7 | 777 | −0.18 (−0.37 to 0.02) | .07 | 35 | 0.23 | .83 |
 Moderated Moderated
|
| LDLf cholesterol | 7 | 734 | −0.08 (−0.23 to 0.07) | .29 | 0 | 0.06 | .95 |
 Moderated Moderated
|
| HDLg cholesterol | 7 | 743 | −0.10 (−0.28 to 0.07) | .24 | 18 | 1.26 | .26 |
 Moderated Moderated
|
| Triglycerides | 7 | 720 | −0.13 (−0.29 to 0.02) | .09 | 0 | 0.21 | .84 |
 Moderated Moderated
|
aSMD: standardized mean difference.
bGRADE: Grading of Recommendations Assessment, Development and Evaluation.
cFBG: fasting blood glucose.
dDowngraded by one level for indirectness (surrogate outcome).
eDowngraded by one level for publication bias.
fLDL: low-density lipoprotein.
gHDL: high-density lipoprotein.
