Figure 3.
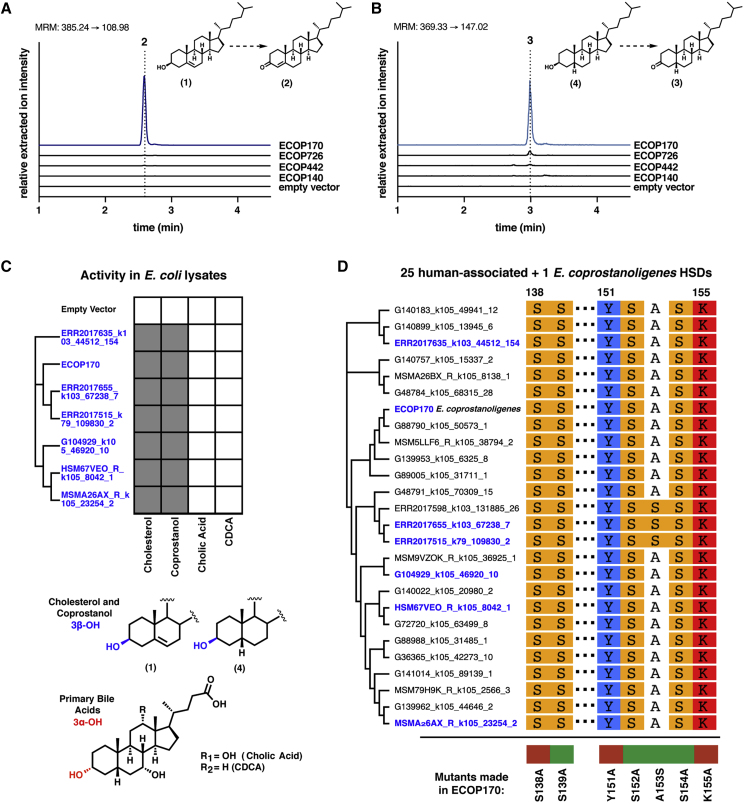
Uncharacterized 3β-Hydroxysteroid Dehydrogenase Enzymes from E. coprostanoligenes and Phylogenetically Related Human-Associated Bacteria Oxidize Cholesterol to Cholestenone
(A and B) (A) A 3β-hydroxysteroid dehydrogenase (3β-HSD) enzyme from E. coprostanoligenes, ECOP170, converts cholesterol (1) to cholestenone (2) and (B) converts coprostanol (4) to coprostanone (3) in the presence of a mixture of 100 μM of NAD+ and 100 μM NADP+.
(C) ECOP170 homologs from gut bacteria heterologously expressed in E. coli convert the 3β-OH groups (blue) of cholesterol and coprostanol (gray squares) to the corresponding ketones (2 and 3, respectively) but were not able to convert primary bile acids, which have 3α-OH groups (red), to the corresponding keto bile acids (white squares). We considered the detection of any of the desired products after overnight incubation in an assay condition to be metabolism.
(D) A multiple sequence alignment of the 25 human-associated cholesterol dehydrogenase homologs and ECOP170 showing the conserved active site residues S138, Y151, and K155. Mutation of any of these residues to alanine in ECOP170 completely abolishes activity (red), whereas proteins with mutations in neighboring residues retain activity (green). Cholesterol dehydrogenases highlighted in blue are confirmed biochemically to oxidize cholesterol.
See also Figures S2–S4 and Tables S2 and S3.