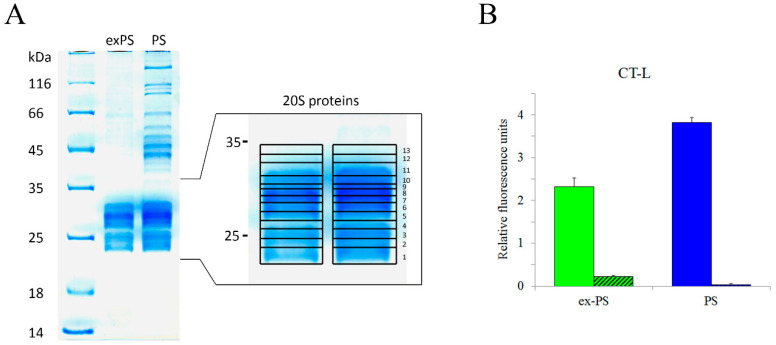
Figure 1.
Affinity-purified extracellular and cellular proteasomes from conditioned medium (CM) and β7-HTBH K562 cells preserve chymotrypsin-like peptidase activity. (A) Proteins from affinity-purified cellular (PS) and extracellular (ex-PS) proteasomes (10 μg) were separated by SDS-PAGE and visualized with Coomassie Blue. Positions of 19S and 20S subcomplexes in the gel are shown. 20S proteasome proteins were cut into 13 pieces, which were then in-gel digested with trypsin. The peptide mixture was analyzed by matrix-assisted laser desorption/ionization Fourier transform ion cyclotron resonance mass spectrometry (MALDI FT-ICR MS). (B) Comparison of the purified intra- and extracellular proteasomes (1 μg) for chymotrypsin-like (CT-L) activity in the presence or absence of proteasome inhibitor MG132, determined by fluorometric quantification of the substrate Suc-LLVY-AMC (N-Succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin), using 380 nm excitation/440 nm emission, respectively. The results are presented in the Y-axis as relative fluorescence units. The control is Suc-LLVY-AMC background fluorescence.