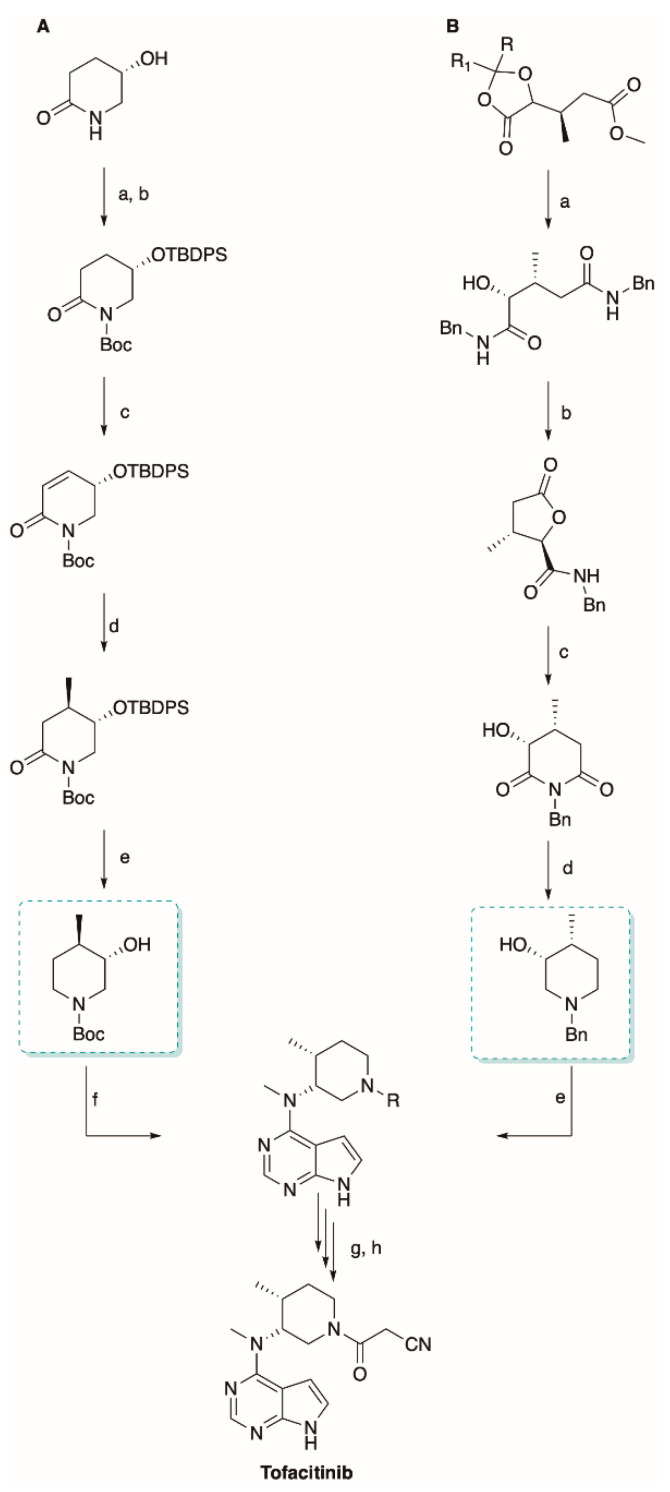
Scheme 5.
The asymmetric synthesis of tofacitinib. Reagents and conditions: (A) (a) TBDPSCl, imidazole, DMF; (b) Boc2O, BuLi, DABCO, THF; (c) (i) HMDS, BuLi, PhSeCl, THF (ii) H2O2; (d) (i) MeMgBr, CuBrSMe3 and (ii) Me3SiCl, Et2O; (e) (i) AlH3, THF and (ii) TBAF/THF; (f) 6-methylamino-7-deazapurine, DIAD-Ph3P, dioxane; (g) ZnBr2, DCM and (h) EDC/HOBt, ethylcyano carboxylic acid, DCM. (B) (a) benzylamine; (b) TFA:H2O (10:1); (c) LDA, THF; (d) LiAlH4, THF and (e) (i) CrO3, H2SO4, AcOH, acetone; (ii) NH2Me, NaBH4, Ti(OiPr)4, MeOH and (iii) 6-chloro-7-deazapurine tosilate, K2CO3, H2O.