Abstract
Background and purpose
Spain has been one of the countries more heavily stricken by SARS‐CoV‐2, which has had huge implications for stroke care. The aim was to analyse the impact of the COVID‐19 epidemic outbreak on reperfusion therapies for acute ischaemic stroke in the northwest of Spain.
Methods
This was a Spanish multicentre retrospective observational study based on data from tertiary hospitals of the NORDICTUS network. All patients receiving reperfusion therapy for ischaemic stroke between 30 December 2019 and 3 May 2020 were recorded, and their baseline, clinical and radiological characteristics, extra‐ and intra‐hospital times of action, Code Stroke activation pathway, COVID‐19 status, reperfusion rate, and short‐term outcome before and after the setting of the emergency state were analysed.
Results
A total of 796 patients received reperfusion therapies for ischaemic stroke. There was a decrease in the number of patients treated per week (46.5 patients per week vs. 39.0 patients per week, P = 0.043) and a delay in out‐of‐hospital (95.0 vs. 110.0 min, P = 0.001) and door‐to‐needle times (51.0 vs. 55.0, P = 0.038). Patients receiving endovascular therapy obtained less successful reperfusion rates (92.9% vs. 86.6%, P = 0.016). COVID‐19 patients had more in‐hospital mortality.
Conclusion
A decrease in the number of patients benefiting from reperfusion therapies was found, with a delay in out‐of‐hospital and door‐to‐needle times and worse reperfusion rates in northwest Spain. COVID‐19 patients had more in‐hospital mortality.
Keywords: acute stroke therapy, cerebral infarction, ischaemic stroke, thrombolysis, Spain, COVID‐19, thrombectomy
Introduction
Since the first reported case in early December 2019, severe acute respiratory coronavirus 2 (SARS‐CoV‐2) infection, known as coronavirus disease 2019 (COVID‐19), has spread all over the world [1, 2, 3]. As of 4 June, more than 6 million cases and 350 000 deaths have been reported worldwide [4]. Treating these patients and containing the outbreak has become the main priority in any centre, running the risk of possible collateral damage on patients with other acute diseases due to the collapse of the pre‐ and intra‐hospital emergency care systems.
Spain has been one of the countries more heavily stricken by SARS‐CoV‐2, with some regions archiving more than 3000 cases per day. In this context, a decrease in the number of hospital admissions for ischaemic strokes has also been described in different regions of the country, in addition to a local report of disruption in their extra‐ and intra‐hospital pathways [5, 6, 7, 8].
The aim of this study was to analyse the impact of the COVID‐19 epidemic outbreak on reperfusion therapies, i.e. intravenous thrombolysis (IVT) and endovascular treatment (EVT), for acute ischaemic stroke in the northwest of Spain. Its impact on hospital admissions, extra‐ and intra‐hospital times of action and short‐term outcome is examined.
Methods
Study design
This Spanish multicentre retrospective observational study was based on the NORDICTUS web‐based prospective reperfusion registry. NORDICTUS is a research and innovation network in cerebrovascular diseases that brings together all public hospitals with stroke units in northwest Spain, with a global catchment area of 11.5 million inhabitants. According to its territorial division, Spain is divided into 17 autonomous communities (AC) and two autonomous cities. Both groups are a first‐order political and administrative division in the country, and NORDICTUS covers eight of them. Sixteen of the 18 referral centres of the network offered their data, including 12 of the 13 centres that perform endovascular treatment in the region. During the pandemic, none of the participating regions has changed its pre‐hospital ischaemic stroke care.
Epidemiological data of COVID‐19 cases were obtained from the Ministry of Health, Consumer Affairs and Social Welfare. Patients with a polymerase chain reaction (PCR) positive for SARS‐CoV‐2 were considered confirmed cases. Due to a change in the counting system in the AC of Galicia, historical data from that administration were obtained from its regional Department of Health (Conselleria de Sanidade) [9, 10].
Study population
All consecutive patients with an ischaemic stroke who received IVT or EVT in tertiary hospitals of the NORDICTUS network between 30 December 2019 and 3 May 2020 were included. Cases were grouped into two periods according to the setting of the state of emergency in Spain (14 March 2020). The start of the COVID‐19 period was considered the eleventh week (W11, 9–15 March) of 2020, due to the political decisions settled that week (travel restrictions, limitations in open‐air events and school closures, until the final lockdown). Demographic and clinical data were recorded including age, sex, vascular risk factors, National Institutes of Health Stroke Scale (NIHSS) score, Alberta Stroke Program Early CT Score (ASPECTS), Code Stroke activation pathway, wake‐up strokes or unknown‐onset time, stroke aetiology, detection of symptoms to door time (DDT), door‐to‐needle time (DNT), door‐to‐puncture time (DPT), puncture to reperfusion time, effective EVT reperfusion [considered as modified treatment in cerebral infarction (mTICI) 2b–3], type of procedural anaesthesia during EVT and COVID‐19 diagnostic test results. As short‐term outcome variables, haemorrhagic transformation after reperfusion therapies and in‐hospital mortality were selected.
COVID‐19 diagnosis was done using PCR techniques of nasopharyngeal swabs for SARS‐CoV‐2.
Statistical analysis
Descriptive statistics were used to compare the incidence of stroke admissions before and after the setting of the state of emergency in Spain, expressed in strokes per week, and the differences between the other study variables in those periods. Qualitative variables are described using counts and percentages, and continuous quantitative variables as means with standard deviation and medians with interquartile ranges, when necessary.
Comparisons between groups were made using chi‐squared tests for comparing categorical variables and the Student test or Mann–Whitney U test for continuous variables. Multiple logistic regression was used to identify factors associated with short‐term outcome variables in those subgroups in which a significant difference was found. P values < 0.05 were considered statistically significant. Statistical analysis was performed with SPSS Statistics 23.0 software (IBM SPSS Statistics 23.0.0.0, New York, NY, USA).
Ethics
The study was approved by the local ethics committee of each participating centre. The treatment of all data obtained in the registry was done following the Spanish data protection law (Data Protection and Digital Rights Guarantee Act).
Results
In total, 796 patients with acute ischaemic stroke (409 men, 51.4%; average age 74.2 years, SD ± 13.0) received reperfusion therapies in the study period: 362 (45.5%) EVT alone, 159 (20.0%) bridging recombinant tissue plasminogen activator (rtPA) before EVT, and 275 (34.5%) only IVT. Table 1 shows the comparison of baseline characteristics of the patients before and after the setting of the state of emergency. There was an increase in the number of patients brought to the hospital by ambulance (46.5% vs. 55.2%, P = 0.019), a worsening in ASPECTS on arrival (10 vs. 9, P = 0.003) and an increase in strokes of undetermined origin (31.2% vs. 39.6%, P = 0.020) after the setting of the state of emergency.
Table 1.
Baseline characteristics of the sample before (pre‐COV) and after (COV) the setting of the state of emergency in Spain.
| pre‐COV (n = 492) | COV (n = 304) | P | |
|---|---|---|---|
| Demographic characteristics | |||
| Sex, male, n (%) | 258 (52.3) | 152 (50.0) | 0.522 |
| Age in years, mean (±SD) | 74.1 (±13.5) | 74.3 (±12.0) | 0.818 |
| Past medical history | |||
| Hypertension, n (%) | 311 (64.7) | 172 (63.0) | 0.649 |
| Diabetes mellitus, n (%) | 105 (21.8) | 56 (20.5) | 0.672 |
| Hyperlipidaemia, n (%) | 242 (50.3) | 143 (52.4) | 0.585 |
| Atrial fibrillation, n (%) | 196 (41.1) | 99 (37.1) | 0.283 |
| Coronary heart disease, n (%) | 64 (13.4) | 28 (10.3) | 0.218 |
| Cerebral infarction, n (%) | 68 (14.2) | 37 (13.6) | 0.807 |
| Peripheral artery disease, n (%) | 32 (6.7) | 15 (5.5) | 0.523 |
| Smoking habit, n (%) | 141 (29.7) | 84 (31.7) | 0.580 |
| Alcohol abuse, n (%) | 40 (8.5) | 19 (7.1) | 0.527 |
| Anticoagulation, n (%) | 80 (16.7) | 35 (12.8) | 0.152 |
| Antiplatelet, n (%) | 135 (28.4) | 66 (24.4) | 0.240 |
| Admission stroke characteristics | |||
| Unknown onset or wake‐up stroke, n (%) | 150 (30.4) | 92 (30.3) | 0.961 |
| Activated by pre‐hospital emergency care, n (%) | 222 (46.5) | 164 (55.2) | 0.019 |
| Transferred from other hospital, n (%) | 128 (26.0) | 85 (28.0) | 0.547 |
| NIHSS score, mean (±SD) | 13.0 (±7.9) | 13.5 (±7.3) | 0.344 |
| ASPECTS, median (IQR) | 10 (8–10) | 9 (8–10) | 0.003 |
| Stroke aetiology | |||
| Atherothrombotic, n (%) | 65 (13.6) | 38 (13.7) | 0.987 |
| Cardioembolic, n (%) | 224 (47.0) | 117 (42.1) | 0.126 |
| Small‐vessel occlusion, n (%) | 12 (2.5) | 5 (1.8) | 0.522 |
| Other, n (%) | 26 (5.5) | 8 (2.9) | 0.099 |
| Undetermined, n (%) | 149 (31.2) | 110 (39.6) | 0.020 |
| COVID‐19‐positive a , n/total | – | 15/158 | – |
ASPECTS, Alberta Stroke Program Early Computed Tomography Score; COVID‐19, coronavirus disease 2019; IQR, interquartile range; NIHSS, National Institutes of Health Stroke Scale; PCR, polymerase chain reaction. Bold font indicates statistical significance.
COVID‐19‐positive: during the COV period, a total of 158 ischaemic stroke patients were tested for SARS‐CoV‐2 infection using PCR techniques.
The number of patients treated with reperfusion therapies in all the participant centres every week decreased once the state of emergency was declared, compared to the number of patients treated before (46.5 patients per week vs. 39.0 patients per week, P = 0.043). For EVT, a decrease was found in those who received general anaesthesia (57.0% vs. 42.4%, P = 0.002) and in the proportion of successful recanalizations (92.9% vs. 86.6%, P = 0.016) (Table 2).
Table 2.
Reperfusion treatment characteristics of the sample before (pre‐COV) and after (COV) the setting of the state of emergency in Spain.
| pre‐COV (n = 492) | COV (n = 304) | P | |
|---|---|---|---|
| Reperfusion treatments per week, median (IQR) | 46.5 (39.8–60.0) | 39 (30.8–45.8) | 0.043 |
| IVT alone, n (%) | 178 (36.2) | 97 (31.9) | 0.218 |
| EVT, n (%) | 314 (63.8) | 207 (68.1) | 0.218 |
| IVT plus EVT, n (%) a | 97 (30.9) | 62 (30.0) | 0.820 |
| Anterior circulation, n (%) a | 259 (88.4) | 169 (89.9) | 0.609 |
| Tandem occlusion, n (%) a | 46 (15.5) | 27 (14.7) | 0.797 |
| General anaesthesia, n (%) a | 175 (57.0) | 81 (42.4) | 0.002 |
| First pass successful reperfusion, n (%) a | 133 (58.3) | 67 (55.4) | 0.595 |
| mTICI 2b–3, n (%) a | 290 (92.9) | 174 (86.6) | 0.016 |
EVT, endovascular treatment; IQR, interquartile range; IVT, intravenous treatment; mTICI, modified treatment in cerebral infarction; rtPA, recombinant tissue plasminogen activator. Bold font indicates statistical significance.
149 patients received bridging rtPA before EVT; bin EVT patients.
In terms of treatment times, there was an increase in DDT (95.0 vs. 110.0 min, P = 0.001) and DNT (51.0 vs. 55.0, P = 0.038), with no significant difference in the other recorded times (Table 3).
Table 3.
Stroke pathway times of the group of patients before (pre‐COV) and after (COV) the setting of the state of emergency in Spain
| pre‐COV (n = 492) | COV (n = 304) | P | |
|---|---|---|---|
| DDT, median (IQR) | 95 (58–179.5) | 110.0 (63.0–217) | 0.043 |
| Inter‐hospital transfer to final hospital | 223 (162.3–295.5) | 251 (205–345) | 0.013 |
| No inter‐hospital transfer | 75.5 (50.0–107.3) | 82.5 (55.0–126.3) | 0.080 |
| DNT, median (IQR) | 51 (36–70) | 55 (43–74) | 0.038 |
| DPT, median (IQR) a | 90 (61.8–115.0) | 91 (64–118) | 0.819 |
| PRT, median (IQR) a | 33 (20–54.0) | 30.0 (18–50.5) | 0.303 |
| DRT, median (IQR) a | 249 (195.8–347) | 273.0 (210–360) | 0.170 |
IQR, interquartile range; DDT, detection of symptoms to final hospital time; DNT, final hospital arrival to needle time; DPT, final hospital arrival to puncture time; PRT, puncture to reperfusion time; DRT, detection of symptoms to reperfusion time. Bold font indicates statistical significance.
In EVT patients; bconsidering successful reperfusion as mTICI 2b–3.
Regarding in‐hospital mortality, no differences were found considering the whole cohort of patients. In the subgroup analysis, only the group of COVID‐19‐positive patients, compared with the COVID‐19‐negative patients (37.5% vs. 9.9%, P = 0.002) (Table S1), and the group of patients who received IVT alone had higher in‐hospital mortality (3.9% vs. 13.4%, P = 0.004) (Table 4). After a multivariate analysis using logistic regression in the latter (adjusted for age and those variables that were significantly different between the two periods), being COVID‐19‐positive was significantly associated with higher in‐hospital mortality (Tables S2 and 5).
Table 4.
Safety and mortality of patients before (pre‐COV) and after (COV) the setting of the state of emergency in Spain.
| Total | EVT (with or without IVT) | IVT alone | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre‐COV | COV | P | Pre‐COV | COV | P | Pre‐COV | COV | P | |
| Asymptomatic HT | 77 (16.2) | 51 (16.9) | 0.789 | 66 (21.3) | 41 (20.0) | 0.724 | 11 (6.7) | 10 (10.4) | 0.283 |
| IH1 | 20 (4.7) | 17 (6.9) | 0.222 | 18 (6.5) | 14 (8.1) | 0.515 | 2 (1.3) | 3 (4.1) | 0.334 |
| IH2 | 15 (3.5) | 10 (4.0) | 0.714 | 14 (5.0) | 8 (4.6) | 0.844 | 1 (0.7) | 2 (2.7) | 0.252 |
| PH1 | 27 (6.3) | 12 (4.9) | 0.441 | 22 (7.9) | 10 (5.8) | 0.391 | 5 (3.3) | 2 (2.7) | 1.000 |
| PH2 | 10 (2.3) | 11 (4.5) | 0.126 | 8 (2.9) | 8 (4.6) | 0.330 | 2 (1.3) | 3 (4.1) | 0.334 |
| Remote or SAH | 22 (5.1) | 12 (4.9) | 0.877 | 18 (6.5) | 10 (5.8) | 0.766 | 4 (2.6) | 2 (2.7) | 1.000 |
| Symptomatic HT | 20 (4.1) | 16 (5.3) | 0.408 | 16 (5.1) | 13 (6.3) | 0.546 | 4 (2.2) | 3 (3.1) | 0.699 |
| In‐hospital mortality | 37 (7.6) | 33 (10.9) | 0.104 | 30 (9.6) | 20 (9.8) | 0.967 | 7 (3.9) | 13 (13.4) | 0.004 |
HT, haemorrhagic transformation; IH, intraparenchymal haematoma; PH, parenchymal haematoma; SAH, subarachnoid haemorrhage. Bold font indicates statistical significance.
Table 5.
Multiple logistic regression of short‐term outcome (in‐hospital mortality) in ‘IVT alone’ group of patients.
| OR (95% CI) | P | |
|---|---|---|
| Age in years | 1.038 (0.969–1.112) | 0.283 |
| Activated by pre‐hospital emergency care | 2.517 (0.411–15.431) | 0.318 |
| ASPECTS | 0.603 (0.406–0.894) | 0.012 |
| Undetermined | 1.016 (0.255–4.043) | 0.982 |
| COVID‐19‐positive | 10.169 (1.840–56.215) | 0.008 |
ASPECTS, Alberta Stroke Program Early Computed Tomography Score; CI, confidence interval; COVID‐19, coronavirus disease 2019; IVT, intravenous thrombolysis; OR, odds ratio.
Bold font indicates statistical significance.
Discussion
There has been a decrease in hospital admissions for ischaemic stroke during the COVID‐19 pandemic in some regions [5, 6, 7, 8, 11]. It has been suggested that possible causes might be changes in social behaviour, minor non‐disabling strokes staying at home, or admission to hospital isolation units where stroke might not be the major issue and might be under‐recognized. There is also a risk that not enough attention has been paid to diagnose it [6]. Some of these factors may have also influenced the number of IVT or EVT in stroke patients, a worrying situation knowing the demonstrated benefit of these treatments on functional prognosis [12, 13, 14]. Data published so far have not shown a decrease in the proportion of reperfusion treatments in admitted ischaemic strokes [6, 8, 11], but these data could be misleading. Considering that there is no reason to think of a real decrease in ischaemic stroke incidence but only in their hospital admissions, maintaining the same proportion of admissions treated with reperfusion therapies means a reduction in the proportion of patients treated from the totality of ischaemic strokes.
In our study, a significant decrease was found in the number of patients with ischaemic stroke who received reperfusion therapy per week in the COVID‐19 period without changes in the type of treatment received (Fig. 1, Table 2). As seen in Fig. 1, in the last two weeks (W17 and W18), there was a rise in the number of reperfusion treatments performed, which seems to correlate with the increment in in‐hospital admissions for ischaemic stroke observed at this date in the study hospitals [6]. This situation could reflect the beginning of a ‘recovery’ period, maybe as a consequence of the different campaigns carried out by the medical staff of most of the centres to raise awareness amongst the population of the importance of attending hospital when detecting stroke symptoms, despite limited free mobility and the risk of catching COVID‐19 infection.
Figure 1.
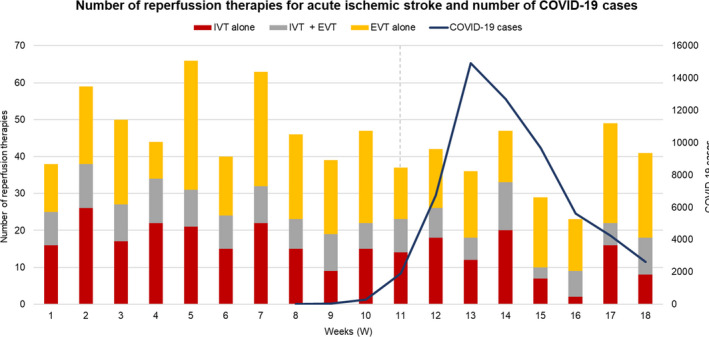
NORDICTUS network: number of reperfusion therapies for acute ischaemic stroke and number of COVID‐19 cases in northwest Spain.
An increase was also found in the proportion of patients who arrived at the hospital by ambulance. This could make sense considering the isolation measures taken by the government to contain the virus dissemination. However, it also means another problem, which is how to deal with the limited availability of ambulance fleets. This saturation of ambulances could explain the delay in the DDT of our patients, a fact especially observed in those transferred from other hospitals.
The increment in DNT may be related to overwhelmed emergency departments and the new circuits established to manage strokes with suspected COVID‐19 during this period. Some examples are the performance of relevant screening questions (respiratory symptoms, potential contact with a confirmed case, travel history), the concern about respiratory status and its management, the PCR testing when a COVID‐19 case is suspected, the use of personal protection equipment, the disposal of potentially contaminated materials, or the sterilization of the contaminated CT control room. This is a new situation for stroke teams that often leads to great uncertainty and anxiety amongst healthcare workers who are in direct contact with patients, and this could have also contributed to the DNT delay.
Given this explanation, the non‐worsening in the DPT of our sample could be because, globally, only a minority of patients who went to EVT received rtPA bridging therapy, so that the delay in DNT did not influence our DPT. Additionally, most of our neurointerventionalists cancelled all scheduled activities and were fully available for emergencies focused on acute stroke treatment, which could have contributed to maintaining these times.
It is believed that pre‐notification continues to be a cornerstone for maintaining good in‐hospital times of care. This will allow patients with high suspicions of SARS‐CoV‐2 infection to be expected with all the protection measures in place. Reinforcing public awareness of stroke warning signs and the importance of getting early to the hospital, even in this pandemic period, could also help improve extra‐hospital attention times.
As other authors have described, higher NIHSS scores in patients treated during the COVID‐19 period were not found [8], but worse ASPECTS results were found. This could be related to the longer out‐of‐hospital times previously described. Moreover, some patients might get out of the treatment group due to baseline characteristics associated with the increment of pre‐hospital attention times.
The decrease observed in the use of general anaesthesia in EVT could obey the application of recommendations in our hospitals to minimize risky procedures such as intubation and avoid aerosol generation. A decrease in the proportion of successful reperfusion (mTICI 2b–3) obtained after EVT was also found. Currently, no study supports the fact that COVID‐19 may be responsible for acute ischaemic stroke with large vessel occlusions or clot composition. So this finding could be related to factors indirectly associated with the COVID‐19 period, such as more organized thrombi due to the delay in out‐of‐hospital times detected in this period.
The relationship between viral infections and ischaemic stroke is well established, with an increased odds of stroke by 1.4‐fold in these patients [16]. Ischaemic strokes occur in about 2% of patients with COVID‐19 infection during hospitalization [17]. A potential link between COVID‐19 and stroke could make sense from a pathophysiological point of view. COVID‐19 can enter vascular endothelium and myocardial cells through angiotensin‐converting enzyme 2 (ACE2) receptor, potentially causing stroke by different mechanisms. Pro‐inflammatory and prothrombotic states associated with COVID‐19 infection could also predispose to thrombogenesis and therefore increase stroke risk. However, evidence of a causal relationship between COVID‐19 and stroke is still limited, including those due to large vessel occlusion. In our sample, the proportion of COVID‐19 patients in those who received reperfusion treatment was low. They had higher in‐hospital mortality than negative COVID‐19 patients (tested by PCR) (Table S1), in accordance with other reports for different diseases and hospital processes that coexist with this infection [18].
In this study, a significant increase in in‐hospital mortality in our patient sample was not found. Nevertheless, this happened in the group of patients who only received IVT, and this higher mortality was independently associated with a higher proportion of COVID‐19‐positive patients in this subgroup. There was an increase in overall mortality in ischaemic strokes attended in our hospitals during the COVID‐19 period, including those who were not candidates for reperfusion therapies [6]. The reasons for this global increase in in‐hospital mortality still need to be elucidated, but amongst other causes it is worth commenting that the hospital overload that occurred in this period could have resulted in worse medical treatment by not admitting all of these patients to stroke units. Moreover, as previously described, stroke patients with COVID‐19 infection may be at an increased risk of in‐hospital mortality due to severe pneumonia, acute respiratory distress syndrome and multi‐organ failure, being cardiovascular and cerebrovascular disease markers of poor outcome in COVID‐19 patients [19].
Finally, it is worth commenting on the increase in strokes of undetermined aetiology in our sample, a situation that could be due to two causes. The first would be related to the hospitals’ collapse by COVID‐19 patients, which even led to the use of neurology inpatient beds for admission of these patients. In the pandemic scenery, the aetiological study of stroke might have been limited to only those strictly necessary tests, without completing the standard study for these patients at discharge, which could have been premature due to the need for beds. The second, more daring to hypothesize, could be due to the fact that ‘the eyes do not see what the mind does not know’, and maybe, in this COVID‐19 period, there have been new or less frequent causes of stroke that have not been taken into account. It may be possible that COVID‐19 infection is related to ischaemic stroke aetiologies that do not fit well into the classical ones. Although it did not reach statistical significance in our sample, 53.3% of COVID‐19‐positive strokes were labelled as of undetermined aetiology versus 34.1% of those that were COVID‐19‐negative (Table S1). The role of hypercoagulability [20], vasculopathy, or even oxygen desaturation and eventual hypoperfusion in the most critically ill patients remains to be elucidated.
Limitations
Our study has some limitations. The main one is inherent to its retrospective, observational nature. Secondly, every centre had its own COVID‐19 testing protocol, and the incidence of COVID‐19 infection amongst all patients with stroke without respiratory symptoms was not investigated, which could have helped an understanding of the highest proportion of strokes of undetermined aetiology during the COVID‐19 period. Besides, other patient characteristics that could correlate with the worse prognosis of COVID‐19 patients, such as the severity of respiratory symptoms or the histopathological thrombus composition in these patients, were not obtained. Finally, the results might not be generalizable to other countries or regions with different stroke care protocols, geographical specificities, and different social and healthcare responses to the COVID‐19 pandemic.
Conclusions
This study offers a perspective about the impact of the COVID‐19 pandemic in reperfusion therapies for acute ischaemic stroke in a vast region, with a global catchment area of 11.5 million inhabitants, in one of the countries more heavily stricken by the SARS‐CoV‐2 epidemic. It shows a decrease in the number of patients treated, with a delay in out‐of‐hospital times and DNTs. For EVT, there was a decline in patients who received general anaesthesia and in successful reperfusion rates. Differences in the proportion of in‐hospital mortality were not found, except for the subgroup of patients who only received IVT and those with COVID‐19 confirmed infection.
Amongst the measures to be taken in the event of a possible second COVID‐19 outbreak, it is necessary to take into account both pre‐ and intra‐hospital scenarios in order to maintain optimal care for patients with acute ischaemic stroke. Furthermore, in our opinion, it would be interesting to establish an operational definition and a standard protocol for the study of cases in which a COVID‐19 infection and a stroke coexist.
Conflicts of interest
JF Arenillas reports having received honoraria as speaker/consultant for the following companies: BI, Pfizer, Daiichi, Bayer, Amgen and Medtronic. E Palacio Portilla reports having received honoraria as speaker/consultant for the following companies: Esteve, Rovi, MSD and AMGEN. The following authors have no conflict of interests: H Tejada Meza, Á Lambea Gil, A Sancho Saldaña, M Martínez‐Zabaleta, E Garmendia Lopetegui, E López‐Cancio Martínez, M Castañón Apilánez, M Herrera Isasi, J Marta Enguita, B Gómez‐Vicente, N Arenaza Basterrechea, JJ Timiraos Fernández, J Sánchez, JL Maciñeiras Montero, M Castellanos‐Rodrigo, D Fernández‐Coud, I Casado Menéndez, MT Temprano Fernández, MM Freijo, A Luna, Y Jiménez López, E Rodríguez Castro, M Rodríguez‐Yáñez, J Tejada García, I Beltrán Rodríguez, F Julián‐Villaverde, MP Moreno García, JM Trejo‐Gabriel‐Galán, A Echavarría Iñiguez, C Pérez Lázaro, MP Navarro, J Marta Moreno.
Supporting information
Table S1. Baseline characteristics, safety and mortality of patients tested for SARS‐CoV‐2 using PCR techniques.
Table S2. Baseline characteristics of the ‘IVT alone’ group (n = 275) before (pre‐COV) and after (COV) the setting of the state of emergency in Spain.
Acknowledgement
Sources of funding: The authors received no financial support for the research, authorship and/or publication of this article.
Nordictus Investigators
Hospital Universitario Miguel Servet: Marta Serrano Ponz, Daniel Sagarra Mur, Rosario Barrena Caballo, Jose María Navasa Melado, Cristina Villar Yus. Hospital Donostia‐Donostia Ospitalea: Ana de Arce Borda, Noemí Diez, Felix Gonzalez, Patricia de la Riva Juez, Jon Rodríguez‐Antigüedad, Jose Angel Larrea, Alex Luttich. Hospital Universitario Central de Asturias: Sergio Calleja, Lorena Benavente, Carmen García‐Cabo, María Rico, Davinia Larrosa, Montserrat González, Pedro Vega, Eduardo Murias, José María Jiménez, Juan Chaviano. Hospital Clínico Universitario Valladolid: Mercedes de Lera Alfonso, Javier Reyes‐Muñoz, Elisa Cortijo García, Ana I. Calleja, María Esther Ramos Araque, Alba Chavarría Miranda, Blanca Talavera de la Esperanza, Isabel Hernández Pérez, Cristina López Sanz, Gonzalo Valle Peñacoba. Hospital Universitario de Araba: Jon Segurola Olaizola, Fernando López Zárraga, Francisco Javier Maynar Moliner. Complejo Hospitalario Universitario A Coruña: Alexia Roel, Sabela Cajaraville, María José Feal, María López‐Fernández. Hospital Universitario de Cabueñes: Celia Antón González. Hospital Universitario de Cruces: T Gonzalez‐Pinto, A Moreno‐Estebanez, I Díaz, T Perez‐Concha, I Ugarriza, E Gonzalez, J Fondevila, I Labayen, J Manso. Hospital Universitario Marqués de Valdecilla: Marian Revilla García, José Luis Martín Gurpegui, José Luis Vázquez Higuera. Complejo Hospitalario Universitario de Santiago: Susana Arias‐Rivas, María Santamaría‐Cadavid, Iria López‐Dequidt, Iago García‐Díaz, Antonio Jesús Mosqueira, José Manuel Pumar. Complejo Asistencial Universitario de León: Ana Fernández Martínez, Sebastián Baldi. Hospital San Pedro: ME Marzo Sola, MA López‐Pérez, M Gómez‐Eguilaz, JM Pérez‐Imbernon, T Martí Tejada. Complejo Asistencial Universitario de Burgos: Yolanda Bravo‐Anguiano, Monica Bartulos‐Iglesias. Hospital Clínico Lozano Blesa: Carlos Tejero Juste, Eduardo Rodriguez Jara.
†The NORDICTUS Investigators are listed in the Appendix.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Cucinotta D, Vanelli M. WHO declares COVID‐19 a pandemic. Acta Biomed 2020; 91: 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fauci AS, Lane HC, Redfield RR. Covid‐19 – navigating the uncharted. N Engl J Med 2020; 382: 1268–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mahase E, Kmietowicz Z. COVID‐19: doctors are told not to perform CPR on patients in cardiac arrest. BMJ 2020; 368: m1282. [DOI] [PubMed] [Google Scholar]
- 4. Situación COVID‐19 en España [Internet]. Madrid: Ministerio de Sanidad, Gobierno de España; February 2020 [access June 4th 2020]. Available at: https://covid19.isciii.es.
- 5. Tejada Meza H, Lambea Gil Á, Sancho Saldaña A, et al. Ischaemic stroke in the time of coronavirus disease 2019. Eur J Neurol. 2020; 27: 1788–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tejada Meza H, Lambea Gil Á, Sancho Saldaña A, et al. Impact of COVID‐19 outbreak in ischemic stroke admissions and in‐hospital mortality in North‐West Spain. Int J Stroke. 2020; 15: 755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rudilosso S, Laredo C, Vera V, et al. Acute stroke care is at risk in the era of COVID‐19. Experience at a comprehensive stroke center in Barcelona. Stroke 2020; 51: 1991–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Montaner J, Barragán‐Prieto A, Pérez‐Sánchez S, et al. Break in the stroke chain of survival due to COVID‐19. Stroke 2020; 51: 2307–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centro Nacional de Epidemiología, Instituto de Salud Carlos III . Datos agregados notificados por las CCAA al Ministerio de Sanidad [Internet Database]. Madrid: Ministerio de Sanidad; February 20th 2020 [upload June 4th 2020; access June 4th 2020]. Available at: https://cnecovid.isciii.es.
- 10. Conselleria de Sanidade, Xunta de Galiza . Datos de notificación de casos en Galicia [Internet]. Santiago de Compostela: Servizo Galego de Saúde. February 2020.
- 11. Zhao J, Li H, Kung D, Fisher M, Shen Y, Liu R. Impact of the COVID‐19 epidemic on stroke care and potential solutions. Stroke 2020; 51: 1996–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group . Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995; 333: 1581–7. [DOI] [PubMed] [Google Scholar]
- 13. Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta‐analysis of individual patient data from randomised trials. Lancet 2014; 384: 1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large‐vessel ischaemic stroke: a meta‐analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 15. Boehme AK, Luna J, Kulick ER, Kamel H, Elkind MSV. Influenza‐like illness as a trigger for ischemic stroke. Ann Clin Transl Neurol. 2018; 5: 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mao L, Wang M, Chen S, He Q, Chang J, Hong C, et al. Neurological manifestations of hospitalized patients with COVID‐19 in Wuhan, China: a retrospective case series study. medRxiv. 2020; 77: 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. COVIDSurg Collaborative . Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS‐CoV‐2 infection: an international cohort study. Lancet 2020; 396: 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pranata R, Huang I, Lim MA, Wahjoepramono EJ, July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID‐19 – systematic review, meta‐analysis, and meta‐regression. J Stroke Cerebrovasc Dis 2020; 29: 104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Terpos E, Ntanasis‐Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID‐19. Am J Hematol. 2020; 95: 834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics, safety and mortality of patients tested for SARS‐CoV‐2 using PCR techniques.
Table S2. Baseline characteristics of the ‘IVT alone’ group (n = 275) before (pre‐COV) and after (COV) the setting of the state of emergency in Spain.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


