Abstract
Aim
To describe the first Australian cases of severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV2) disease (COVID‐19) pneumonia treated with the interleukin‐6 receptor antagonist tocilizumab.
Methods
Retrospective, open‐label, real‐world, uncontrolled, single‐arm case series conducted in 2 tertiary hospitals in NSW, Australia and 1 tertiary hospital in Victoria, Australia. Five adult male patients aged between 46 and 74 years with type 1 respiratory failure due to COVID‐19 pneumonia requiring intensive care unit (ICU) admission and biochemical evidence of systemic hyperinflammation (C‐reactive protein greater than 100 mg/L; ferritin greater than 700 μg/L) were administered variable‐dose tocilizumab.
Results
At between 13 and 26 days follow‐up, all patients are alive and have been discharged from ICU. Two patients have been discharged home. Two patients avoided endotracheal intubation. Oxygen therapy has been ceased in three patients. Four adverse events potentially associated with tocilizumab therapy occurred in three patients: ventilator‐associated pneumonia, bacteremia associated with central venous catheterization, myositis and hepatitis. All patients received broad‐spectrum antibiotics, 4 received corticosteroids and 2 received both lopinavir/ritonavir and hydroxychloroquine. The time from first tocilizumab administration to improvement in ventilation, defined as a 25% reduction in fraction of inspired oxygen required to maintain peripheral oxygen saturation greater than 92%, ranged from 7 hours to 4.6 days.
Conclusions
Tocilizumab use was associated with favorable clinical outcome in our patients. We recommend tocilizumab be included in randomized controlled trials of treatment for patients with severe COVID‐19 pneumonia, and be considered for compassionate use in such patients pending the results of these trials.
Keywords: acute respiratory distress syndrome, coronavirus, immunomodulation, interleukin‐6, pneumonia, tocilizumab, viral
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), caused by the severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2), was declared a pandemic by the World Health Organization (WHO) on 11 March 2020. As at 24 May 2020 there have been 7135 cases of COVID‐19 confirmed in Australia, of which 947 (13%) have been hospitalized, 202 (21%) required intensive care unit (ICU) admission and 102 have died. 1
Twenty percent of COVID‐19 cases can develop acute respiratory distress syndrome (ARDS), which confers up to 62% mortality. 2 , 3 Despite low rates of severe disease in Australia, 4 therapies for COVID‐elated ARDS are urgently needed.
There is emerging evidence that COVID‐19‐related ARDS is triggered by excessive secretion of pro‐inflammatory signaling molecules, termed cytokine release syndrome (CRS). 2 While there is no consensus definition of COVID‐19 CRS, current data suggest it is clinically characterized by rapid‐onset respiratory failure and biochemically characterized by raised interleukin‐6 (IL‐6) and its surrogate markers, including C‐reactive protein (CRP) and ferritin. 5 , 6
Tocilizumab, a humanized monoclonal antibody targeting the IL‐6 receptor (IL‐6R), was approved by the Therapeutic Goods Administration for the treatment of rheumatoid arthritis in Australia in 2009. 7 Established uses have since expanded to include juvenile idiopathic arthritis (JIA), giant cell arteritis, chimeric antigen receptor (CAR) T‐cell therapy CRS and secondary hemophagocytic lymphohistiocytosis (sHLH) related to JIA, also termed macrophage activation syndrome. 8 Although tocilizumab is generally well tolerated in patients with rheumatic diseases, serious infections are reported at a rate of 4.7 events per 100 patient‐years of use, including bacterial, viral and opportunistic infections, some of which have been fatal. 7 Tocilizumab is relatively contraindicated in patients with active infection. 7 , 9
Some clinical and laboratory findings in COVID‐19 CRS are similar to those found in sHLH and CRS induced by CAR T‐cell therapy. 2 , 10 However, it is not yet known whether IL‐6, which is implicated as causative in the latter two conditions 2 , 11 plays a similar role in COVID‐19 CRS. 8 , 11 IL‐6 is also raised in severe sepsis, 12 but data related to tocilizumab in sepsis are limited to in vitro and animal studies, and other immune interventions in sepsis using tumor necrosis factor alpha and interleukin‐1 antagonists have not been associated with improved outcomes. 13 , 14
Following the publication of two small, uncontrolled, retrospective case series from China, 15 there has been considerable interest in tocilizumab use in patients with COVID‐19. 2 , 10 , 16 Two case control series reporting positive short‐term outcomes have now been published in the peer‐reviewed scientific literature, 17 , 18 and two additional case control series have been published to the preprint server medRxiv but are yet to undergo peer review. 19 , 20 We describe our experience using tocilizumab in patients with severe COVID‐19 pneumonia.
2. MATERIALS AND METHODS
Between 30 March 2020 and 11 April 2020, 5 patients with clinical deterioration in the context of a systemic inflammatory response to COVID‐19 were treated with tocilizumab at three tertiary referral hospitals, 2 in NSW and 1 in Victoria. All patients had confirmed SARS‐CoV2 infection based on real‐time polymerase chain reaction analysis of nasal swab sample, radiological findings consistent with COVID‐19 pneumonia and biochemical evidence of systemic inflammation with CRP greater than 100 mg/L (NR [normal range] ≤5 mg/L) and ferritin greater than 700 μg/L (NR 30‐400 μg/L). At the time of tocilizumab administration, three patients met the Berlin ARDS definition, 21 had undergone endotracheal intubation and were mechanically ventilated due to type 1 respiratory failure. The other 2 patients had rapid, progressive type 1 respiratory failure but did not meet the Berlin ARDS definition as continuous positive airway pressure was not applied due to concern for aerosolization of SARS‐CoV2. The decision to treat with tocilizumab was made by consensus between the involved intensive care, respiratory, infectious diseases and immunology specialists. Informed consent was obtained from the patient in two cases and from the next of kin in the three intubated patients. Clinical information for each patient was obtained from a review of electronic and paper medical record systems, from which sequential organ failure assessment (SOFA) score 22 and H‐score 23 were calculated where possible. Ethics approval was not required at two sites and was obtained at one site. All patients consented to publication.
3. RESULTS
The five patients were aged between 46 and 74 years and were followed for between 13 and 26 days after tocilizumab therapy; see Figure 1. Table 1 describes patient demographics, past medical history and time‐course of events prior to tocilizumab administration. All patients additionally received broad‐spectrum antibiotics; four patients received corticosteroids; and two received both hydroxychloroquine and lopinavir/ritonavir (LPV/r). The time from tocilizumab administration to improvement in oxygenation, defined as a 25% fall in fraction of inspired oxygen (FiO2) required to maintain peripheral oxygen saturation (SpO2) greater than 92%, ranged from 7 hours to 4.6 days; Table 2 describes tocilizumab dose, additional medications administered, progress following treatment and adverse events. Table 3 and Figure 2 describe clinical and laboratory results before and after tocilizumab treatment, and in Table S1 the Supplementary Appendix details further laboratory parameters.
Figure 1.
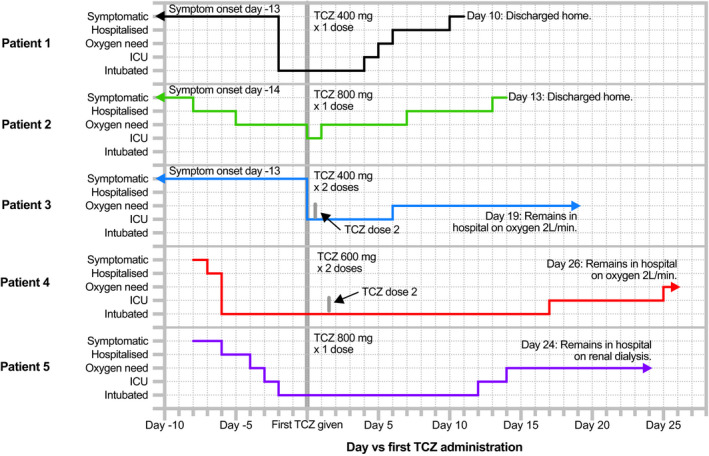
Timeline of clinical progress of COVID‐19 patients before and after tocilizumab treatment. COVID‐19, coronavirus disease 2019; ICU, intensive care unit; L/min, liters per minute; TCZ, tocilizumab
Table 1.
Patient background, time‐course of admission and assessment prior to tocilizumab administration
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Demographic data and medical background | |||||
| Age (y) | 53 | 74 | 71 | 70 | 46 |
| Gender | Male | Male | Male | Male | Male |
| Weight (kg) | 70 | 90 | 68 | 72 | 86 |
| BMI (kg/m2) | 25.1 | 27.2 | 23.0 | 26.5 | 32.8 |
| Smoker | No | No | No | No | No |
| Ethnicity | Chinese | Caucasian | Caucasian | Indian | Filipino |
| Coexisting chronic diseases | Hypertension, dyslipidemia | Type II diabetes mellitus, hypertension | Dyslipidemia, aortic valve replacement, lacunar stroke | Type II diabetes mellitus | Asthma, obesity |
| Regular medications | Rosuvastatin, telmisartan | Allopurinol, aspirin, gliclazide MR, irbesartan, metformin, rosuvastatin | Aspirin, pantoprazole, ramipril, rosuvastatin, warfarin | Metformin | Salbutamol as‐needed |
| Time‐course from day of symptom onset | |||||
| Event | Days later | Days later | Days later | Days later | Days later |
| SARS‐CoV2 swab + ve | 2 | 3 | 1 | 2 | 0 |
| Hospital admission | 11 | 6 | 13 | 2 | 3 |
| O2 requirement started | 11 | 9 | 13 | 3 | 5 |
| ICU admission | 11 | 14 | 13 | 3 | 6 |
| Intubation | 11 | (Not intubated) | (Not intubated) | 3 | 7 |
| TCZ administration | 11 | 14 | 13 | 9 | 9 |
| Assessment prior to TCZ | |||||
| PF ratio (mm Hg) | 124 | 198 | 93 | 112 | 69 |
| SOFA score | 6 | 2 | 2 | 7 | 14 |
| H‐score | Insufficient data | Insufficient data | Insufficient data | 151 | 132 |
| Infection screen | |||||
| When tested vs TCZ | 4 d prior | 2 h after | 3 h prior | 11 h prior | Not tested |
| QuantiFERON Gold | Indeterminate | Indeterminate | Indeterminate | Not tested | Not tested |
| Hepatitis B serology | Vaccinated | Negative | Negative | Negative | Not tested |
| HIV serology | Negative | Negative | Negative | Negative | Not tested |
Abbreviations: BMI, body mass index; HIV, human immunodeficiency virus; ICU, intensive care unit; MR, modified release; O2, oxygen; PF ratio, ratio of partial pressure of oxygen in arterial blood to fraction of inspired oxygen; SARS‐CoV2, severe acute respiratory syndrome‐coronavirus 2; SOFA, sequential organ failure assessment; TCZ, tocilizumab.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Table 2.
Tocilizumab treatment, other treatments received and response to tocilizumab administration
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| TCZ dose | 400 mg IV x 1 dose | 800 mg IV x 1 dose | 400 mg IV x 2 doses 8 h apart | 600 mg IV x 2 doses 39 h apart | 800 mg x IV 1 dose |
| Medication | Dose and duration | Dose and duration | Dose and duration | Dose and duration | Dose and duration |
|---|---|---|---|---|---|
| Other treatments received | |||||
| Corticosteroids | MP 1 mg/kg IV daily x 2 doses from d of TCZ | P 25 mg/d tapered to cessation from 5 d after TCZ | No | P 25 mg/d x 3 d then 75 mg/d x 3 d finishing on d of hospitalization | MP 1 g x 1 dose, 100 mg/d x 3 d, 40 mg x 3 d, 5 mg x 1 d from d of TCZ |
| Antiretrovirals | LPV/r 400 mg/ 100 mg BD x 6 d from d of TCZ | No | LPV/r 400 mg/ 100 mg BD x 3 d from d of TCZ | No | No |
| Antimalarial | HCQ 200 mg BD x 6 d from d of TCZ | No | HCQ 200 mg BD x 6 d from d of TCZ | No | No |
| Event | Days later | Days later | Days later | Days later | Days later |
|---|---|---|---|---|---|
| Timing from d of TCZ to key clinical events | |||||
| Extubation | 4 | (Not intubated) | (Not intubated) | 17 | 12 |
| ICU discharge | 5 | 1 | 6 | 24 | 14 |
| O2 therapy cessation | 6 | 7 | Not yet achieved | Not yet achieved | 14 |
| Hospital discharge | 10 | 13 | Not yet achieved | Not yet achieved | Not yet achieved |
| Final day of follow‐up | 25 | 13 | 19 | 26 | 24 |
| Criterion | Time vs TCZ | Time vs TCZ | Time vs TCZ | Time vs TCZ | Time vs TCZ |
|---|---|---|---|---|---|
| Response to TCZ administration | |||||
| Defervescence (fever < 38°C) | Immediate | 3 d prior to TCZ | Immediate | Immediate | 5 h |
| FiO2 fall > 25% | 4.6 d | 15 h | 50 h | 4.0 d | 7 h |
| CRP fall > 25% | 37 h | 46 h | 35 h | 18 h | 31 h |
| CRP fall > 50% | 37 h | 46 h | 35 h | 41 h | 31 h |
| Ferritin fall > 25% | 62 h | 22 h | Not achieved | 41 h | 55 h |
| Ferritin fall > 50% | Not achieved | Not achieved | Not achieved | 17.6 d | 10.3 d |
| Radiographic improvement | 41 h | 9.8 d | 10.2 d | 7.1 d | 3.3 d |
| Adverse events | Ventilator‐associated pneumonia. | Nil. | Hepatitis. | Delirium. | Renal failure, myositis, line‐associated Staphylococcus epidermidis bacteremia, eosinophilia. |
Abbreviations: BD, twice daily; CRP, C‐reactive protein; FiO2, fraction of inspired oxygen; HCQ, hydroxychloroquine; ICU, intensive care unit; IV, intravenous; LPV/r, oral lopinavir/ritonavir; MP, intravenous methylprednisolone; O2, oxygen; P, oral prednisolone; PO, per oral; TCZ, tocilizumab.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Table 3.
Clinical and laboratory parameters of COVID‐19 patients before and after TZC treatment
| Case No. | Before TCZ therapy | Day of TCZ therapy | Days after TCZ therapy | Clinical outcome | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day of hospitalization | Day of ICU admission | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 10 | ||||
| Ventilation and oxygenation | |||||||||||||
| 1 | Time vs TCZ | 2 d prior | Same day | Discharged home d 10 post‐TCZ. | |||||||||
| O2 delivery method | ETT | ETT | ETT | ETT | ETT | NP | NP | NP | RA | RA | RA | ||
| FiO2 (%) | 80 | 30 | 30 | 30 | 35 | 36 | 36 | 28 | 21 | 21 | 21 | ||
| PF ratio (mm Hg) | 124 | 253 | 253 | 253 | 209 | 226 | 306 | 286 | |||||
| 2 | Time vs TCZ | 8 d prior | Same day | Discharged home d 13 post‐TCZ. | |||||||||
| O2 delivery method | RA | HM | HM | NP | NP | NP | NP | NP | NP | NP | RA | ||
| FiO2 (%) | 21 | 60 | 60 | 36 | 36 | 36 | 36 | 32 | 28 | 28 | 21 | ||
| PF ratio (mm Hg) | 198 | 198 | 277 | ||||||||||
| 3 | Time vs TCZ | Same day | Same day | D 19 post‐TCZ: remains in hospital on ward bed on oxygen 2 L/min. | |||||||||
| O2 delivery method | HM | HM | HM | NP | NP | NP | NP | NP | NP | NP | NP | ||
| FiO2 (%) | 60 | 60 | 60 | 36 | 36 | 36 | 36 | 36 | 36 | 36 | 28 | ||
| PF ratio (mm Hg) | 93 | 93 | 93 | 277 | |||||||||
| 4 | Time vs TCZ | 7 d prior | 6 d prior | D 26 post‐TCZ: remains in hospital on ward bed on oxygen 2 L/min. | |||||||||
| O2 delivery method | RA | ETT | ETT | ETT | ETT | ETT | ETT | ETT | ETT | ETT | ETT | ||
| FiO2 (%) | 21 | 45 | 55 | 60 | 70 | 60 | 40 | 40 | 30 | 40 | 40 | ||
| PF ratio (mm Hg) | 342 | 112 | 97 | 81 | 95 | 165 | 135 | 186 | 140 | 165 | |||
| 5 | Time vs TCZ | 6 d prior | 2 d prior | D 24 post‐TCZ: remains in hospital on ward bed, breathing room air, on renal dialysis. | |||||||||
| O2 delivery method | RA | HM | ETT | ETT | ETT | ETT | ETT | ETT | ETT | ETT | ETT | ||
| FiO2 (%) | RA | 30 | 100 | 45 | 50 | 35 | 35 | 35 | 30 | 35 | 30 | ||
| PF ratio (mm Hg) | 183 | 69 | 193 | 128 | 314 | 197 | 171 | 213 | 200 | 267 | |||
| CRP (mg/L; NR ≤ 4.9) | |||||||||||||
| 1 | 227 | 236 | 236 | 216 | 86 | 33 | 16 | 4 | 3 | ||||
| 2 | 82 | 128 | 128 | 37 | 17 | 12 | 9 | 6 | |||||
| 3 | 269 | 269 | 269 | 236 | 86 | 40 | 21 | 11 | 7 | 2 | |||
| 4 | 91 | 226 | 530 | 389 | 164 | 73 | 40 | 24 | 17 | 17 | 12 | ||
| 5 | 87 | 105 | 312 | 319 | 177 | 89 | 23 | 6 | 3 | ||||
| Ferritin (μg/L; NR 30‐400) | |||||||||||||
| 1 | 4700 | 4396 | 4396 | 4501 | 2684 | 2670 | |||||||
| 2 | 259 | 738 | 738 | 545 | 470 | 379 | 457 | 420 | 420 | ||||
| 3 | 731 | 731 | 731 | 774 | 865 | 812 | 762 | 681 | 669 | 546 | |||
| 4 | 3177 | 2666 | 2296 | ||||||||||
| 5 | 2080 | 2074 | 1611 | 1501 | |||||||||
| LDH (U/L; NR ≤ 250) | |||||||||||||
| 1 | 471 | 362 | 362 | 466 | 288 | 394 | |||||||
| 2 | 255 | 255 | 213 | 221 | 227 | 231 | 202 | ||||||
| 3 | 731 | 731 | 417 | 309 | 328 | 281 | 254 | 291 | 230 | 270 | |||
| 4 | 324 | 303 | 280 | ||||||||||
| 5 | 705 | 541 | |||||||||||
Abbreviations: COVID‐19, coronavirus disease 2019; CRP, C‐reactive protein; ETT, endotracheal tube; FiO2, fraction of inspired oxygen; HM, Hudson mask; ICU, intensive care; LDH, lactate dehydrogenase; NP, nasal prongs; NR, normal range; O2, oxygen; PF ratio, ratio of partial pressure of oxygen in arterial blood to fraction of inspired oxygen; RA, room air; TCZ, tocilizumab; U/L, units per liter.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Figure 2.
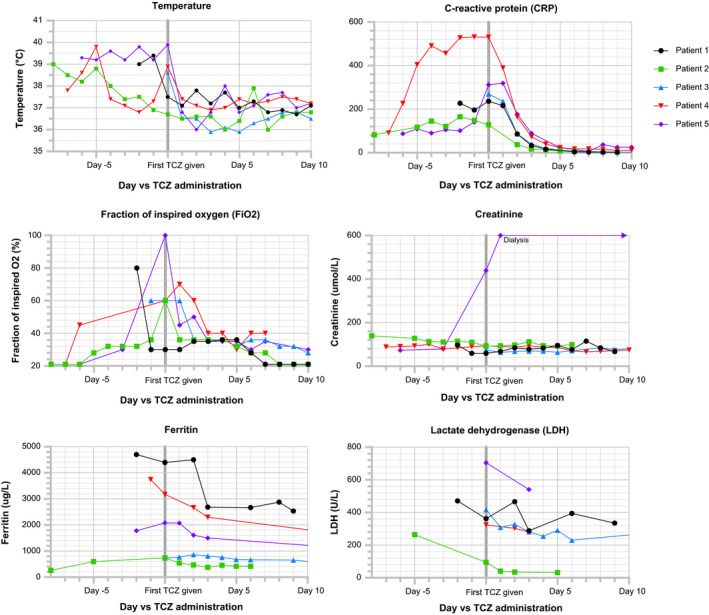
Clinical and laboratory parameters of COVID‐19 patients before and after TCZ treatment. COVID‐19, coronavirus disease 2019; TCZ, tocilizumab
3.1. Patient 1
A 53‐year‐old man with a history of hypertension presented to hospital with fever and rapid‐onset type I respiratory failure due to COVID‐19 pneumonia. At presentation, partial pressure of oxygen in arterial blood (PaO2) was 99 mm Hg while receiving FiO2 80% yielding PaO2 to FiO2 ratio (PF ratio) of 93 mm Hg. He underwent endotracheal intubation and was mechanically ventilated using the ARDSNet protocol 24 to manage adequate gas exchange and noradrenaline to maintain mean arterial blood pressure greater than 70 mm Hg. A total of 6 days LPV/r and hydroxychloroquine were administered commencing at day 2 of hospitalization. On day 3 of hospitalization, intravenous (IV) tocilizumab 400 mg and methylprednisolone 70 mg (1 mg/kg) were administered. Noradrenaline was ceased 6 hours after tocilizumab administration. Two days after tocilizumab therapy, ventilatory function improved, but signs of encephalopathy arose when sedation was weaned. Corticosteroids were therefore ceased and encephalopathy resolved rapidly. Ventilator‐associated pneumonia, diagnosed 3 days after tocilizumab administration, was treated with piperacillin/tazobactam. Three days following tocilizumab administration, the patient was extubated. He was discharged from ICU 5 days after tocilizumab administration, ceased oxygen therapy 1 day later, and was discharged home after a further 4 days.
3.2. Patient 2
A 74‐year‐old man with a history of type II diabetes and hypertension presented to hospital with COVID‐19 pneumonia, with SpO2 95% on room air at presentation. He became hypoxic on admission day 3, and oxygen therapy, ceftriaxone and azithromycin were commenced. On day admission 9 he developed rapid‐onset type I respiratory failure with clinical and biochemical features of COVID‐19 CRS. PaO2 was 119 mm Hg while receiving oxygen 8 L/min via Hudson mask (estimated FiO2 60%) yielding a PF ratio of 198 mm Hg. He was admitted to ICU and tocilizumab 800 mg IV was administered. Following tocilizumab administration, estimated FiO2 requirement fell from 60% to 36% in 16 hours while maintaining SpO2 greater than 92%. He did not require endotracheal intubation. He was discharged from ICU 20 hours after tocilizumab administration. He then remained stable on FiO2 36% for 5 days, when oral prednisone 25 mg daily was commenced. Oxygen therapy was ceased 7 days after tocilizumab administration, and he was discharged home 6 days later. Corticosteroids were tapered to cessation over 2 weeks.
3.3. Patient 3
A 71‐year‐old man with a history of bioprosthetic aortic valve replacement and lacunar stroke presented to hospital with fever and acute type I respiratory failure due to COVID‐19 pneumonia. PaO2 was 56 mm Hg while receiving oxygen 8 L/min via Hudson mask (estimated FiO2 60%) yielding a PF ratio of 93 mm Hg. He was immediately admitted to ICU and 2 doses of 400 mg tocilizumab were administered 8 hours apart. FiO2 requirement reduced to 36% while maintaining SpO2 greater than 92% 50 hours after first tocilizumab administration. He did not require endotracheal intubation and complied with conscious proning for 16 hours per day. He received 3 days of LPV/r and 6 days of hydroxychloroquine; all were ceased due to prolonged corrected QT interval on electrocardiogram. He was discharged from ICU 6 days after tocilizumab administration. Twelve days after tocilizumab administration he developed hepatitis, which resolved over 5 days without specific intervention. A screen for infectious and autoimmune hepatitis was negative. Abdominal ultrasound demonstrated hepatic steatosis. Gastroenterology opinion was of either drug‐induced liver injury or COVID‐19 hepatitis. On day 19 after tocilizumab administration he remained admitted to hospital, requiring oxygen via nasal cannulae.
3.4. Patient 4
A 70‐year‐old man with a history of recently diagnosed type II diabetes presented to hospital with acute type I respiratory failure due to COVID‐19 pneumonia, with PF ratio of 343 mm Hg and CRP of 91 mg/L at hospital admission. Intravenous ceftriaxone and azithromycin were commenced. On day 2 of hospitalization, he was admitted to ICU, underwent endotracheal intubation and commenced mechanical ventilation. Noradrenaline was administered to maintain mean airway pressure >70 mm Hg. By admission day 7, PF ratio was 112 mm Hg and CRP was 530 mg/L. Starting on admission day 7, 2 doses of tocilizumab 600 mg (8 mg/kg) were administered 39 hours apart and IV piperacillin/tazobactam was commenced. Following tocilizumab dose 1, he remained critically unwell for 3 days. His PF ratio improved to 185 mm Hg on day 4 following the first dose of tocilizumab and noradrenaline was ceased 1 day later. Seventeen days after tocilizumab, his PF ratio was 303 mm Hg and he was extubated. Twenty‐four days after tocilizumab he was discharged from ICU, and on day 26 after tocilizumab administration he remained admitted to hospital, receiving oxygen via nasal cannulae. Fluctuating delirium has been his only complication.
3.5. Patient 5
A 46‐year‐old man with a history of obesity and asthma presented to hospital with COVID‐19 pneumonia, with SpO2 95% on room air at presentation. He developed type I respiratory failure on admission day 3 and underwent endotracheal intubation and mechanical ventilation on day 4. He developed acute kidney injury on admission day 5 requiring continuous renal replacement therapy by day 6. On day 6, PF ratio was 69 mm Hg and there were clinical and biochemical features of CRS. Manual proning was instituted, and a single dose of tocilizumab 800 mg IV and 1 g of IV methylprednisolone were administered, followed by IV methylprednisolone 100 mg daily for 3 days tapered to cessation over four further days. PF ratio improved to 193 mm Hg the day after tocilizumab administration, and he was extubated 11 days later. Oxygen therapy was ceased 15 days after tocilizumab administration.
His admission was complicated by myositis of uncertain etiology. Magnetic resonance imaging of the spine on day 17 post‐tocilizumab demonstrated extensive inflammation of the left psoas and iliacus muscles and surrounding the left lumbar plexus. He further suffered Staphylococcus epidermidis bacteremia attributed to central venous catheterization, treated with IV vancomycin and venous catheter removal, and developed an eosinophilia (peak 1.9 × 10⁹ cells/L) for which investigations are ongoing. At 24 days after tocilizumab administration, he remains admitted to hospital and dialysis‐dependent. Renal biopsy is being considered.
4. DISCUSSION
We present the first Australian cases of tocilizumab use in COVID‐19, comprising five patients with severe COVID‐19 pneumonia and clinical suspicion for CRS. Our retrospective, uncontrolled, open‐label, variable protocol, real‐world evidence series should be interpreted with caution. Nevertheless, we believe it is vital that such cases are published to inform current debate and to generate clinical hypotheses about mechanisms of disease and the potential for interventions.
At 13 to 26 days follow‐up of our five patients, 2 had been discharged home and 3 had left ICU but remained hospitalized. Three of 5 patients had ceased oxygen therapy. Where used, corticosteroids, LPV/r and hydroxychloroquine could have contributed to these outcomes. Further, there were several adverse events, including 1 with patient bacterial ventilator‐associated pneumonia, hepatitis, myositis and bacteremia attributed to central venous catheterization. Tocilizumab could be implicated in these events, but these findings are not uncommon in the critically ill and we await the results of randomized controlled trials to clarify the safety of tocilizumab in this population 15 ; LPV/r could also have contributed to hepatitis.
In the face of a rapidly expanding pandemic and when evidence‐based therapies or randomized trials of novel treatments are not available, it may be ethical to offer treatments with biologic plausibility and an established role in analogous diseases on a compassionate basis to patients in specific circumstances. For example, in the United States, both the Food and Drug Administration (FDA) 25 and American Medical Association (AMA) 25 state that compassionate use may be considered if:
There is no comparable or satisfactory therapy available,
the probable risk from the investigational product is not greater than the probable risk from the disease, and
providing the investigational product will not interfere with the conduct of clinical trials.
The significant mortality of severe COVID‐19 pneumonia provides further justification for administration of biologically plausible but unproven treatments within these parameters; this argument was also employed during the 2014‐2015 Ebola outbreak. 26
Of central importance is whether the inflammatory response to COVID‐19 is an adaptive response to severe infection, and when, if at all, pathogenic hyperinflammation occurs. Raised levels of IL‐6 have been shown to predict poor outcomes in severe COVID‐19 infection, and our data add to the published literature suggesting IL‐6 pathway inhibition may be beneficial. 11 However, IL‐6 levels are also elevated in sepsis, 12 where immune interventions have failed to show benefit. 13 Further research into the immune response to SARS‐CoV2 infection is required.
Should Level 1 evidence for tocilizumab in COVID‐19 CRS emerge, patient selection and timing of drug administration will become crucial questions. Several protocols have been published, although none are backed by robust clinical data. 27 , 28 Our results can be regarded only as hypothesis‐generating. Physicians considering the potential value of tocilizumab in COVID‐19 in absence of robust evidence should consider whether the risk of benefit outweighs the risk of harm, particularly in view of the known roles of IL‐6 in host defense to viruses and the lack of a clear demarcation between adaptive and maladaptive inflammatory immune responses to infection. Treatment in mild or early infection could result in suppression of beneficial, antiviral effects of IL‐6‐mediated inflammation, whereas treatment too late may be ineffective.
The dose and schedule of tocilizumab used in our series varied between patients, and there is treatment heterogeneity in the published literature. 15 If tocilizumab is shown to be effective for COVID‐19 CRS, phase 2 dosing studies will be required. If global supply comes under strain, the needs of both COVID and non‐COVID patients for tocilizumab will need to be considered.
In our series, the time from tocilizumab administration to an improvement in oxygenation, defined as a 25% fall in FiO2 requirement, ranged from 7 hours to 4.6 days, and radiographic improvement occurred at between 41 hours and 9.8 days. While no laboratory parameter reliably predicted clinical improvement in all five patients, changes in ferritin and lactate dehydrogenase generally correlated with clinical improvement, whereas D‐dimer, creatine kinase, troponin‐T and neutrophil counts did not. While all patients rapidly defervesced and CRP rapidly normalized, these are IL‐6‐dependent processes, 29 and so are unlikely to predict clinical improvement.
This series illustrates that tocilizumab can be associated with favorable clinical outcome in some cases of severe COVID‐19 pneumonia. The Australian Society for Clinical Immunology and Allergy (ASCIA) recommends IL‐6 blockade be considered early in critically ill patients with severe COVID‐19 pneumonia. 9 We concur with these recommendations and advocate for collaborative, multidisciplinary management of such patients, including judicious use of tocilizumab, while awaiting the results of large multicenter placebo‐controlled randomized trials.
CONFLICT OF INTERESTS
The authors have no conflicts of interest to declare.
ETHICAL APPROVAL
Ethics approval was obtained from the Monash Health Human Research Ethics Committee. All patients had an opportunity to review the manuscript and consented to their cases being published.
Supporting information
Table S1
West TA, Malik S, Nalpantidis A, et al. Tocilizumab for severe COVID‐19 pneumonia: Case series of 5 Australian patients. Int J Rheum Dis. 2020;23:1030–1039. 10.1111/1756-185X.13913
DATA AVAILABILITY STATEMENT
All authors had full access to all of the data in the study.
REFERENCES
- 1. Australian Government .COVID‐19, Australia: Epidemiology Report 17: Fortnightly reporting period ending 24 May 2020. Canberra, ACT: Department of Health: Australian Government; 2020 5/6/2020. [Google Scholar]
- 2. Moore BJB, June CH. Cytokine release syndrome in severe COVID‐19. Science. 2020;368(6490):473‐474. [DOI] [PubMed] [Google Scholar]
- 3. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Australian Government .COVID‐19, Australia, Epidemiology Report 13: Reporting week ending 23:59 AEST 26 April 2020. Canberra, ACT: Department of Health: Australian Government; 2020. 1/5/2020. [Google Scholar]
- 5. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Australian Product Information . Tocilizumab. Sydney, Australia: Roche Products Pty Limited; 2020. 27/3/2020.
- 8. Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting Interleukin‐6 Signaling in Clinic. Immunity. 2019;50(4):1007‐1023. [DOI] [PubMed] [Google Scholar]
- 9. Australian Society for Clinical Immunology and Allergy (ASCIA) . Position Statement ‐ Specific Treatments for COVID‐19. Sydney, NSW: Australian Society for Clinical Immunology and Allergy (ASCIA); 2020. 20/4/2020 (date of last content update). [Google Scholar]
- 10. Ascierto PA, Fox B, Urba W, et al. Insights from immuno‐oncology: the Society for Immunotherapy of Cancer Statement on access to IL‐6‐targeting therapies for COVID‐19. J Immunother Cancer. 2020;8(1):e00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herold T, Jurinovic V, Arnreich C, et al. Level of IL‐6 predicts respiratory failure in hospitalized symptomatic COVID‐19 patients. medRxiv preprint server. 2020;2020:04.01.20047381. [Google Scholar]
- 12. Kruttgen A, Rose‐John S. Interleukin‐6 in sepsis and capillary leakage syndrome. J Interferon Cytokine Res. 2012;32(2):60‐65. [DOI] [PubMed] [Google Scholar]
- 13. Peters van Ton AM, Kox M, Abdo WF, Pickkers P. Precision immunotherapy for sepsis. Front Immunol. 2018;9:1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ibrahim YF, Moussa RA, Bayoumi AMA, Ahmed AF. Tocilizumab attenuates acute lung and kidney injuries and improves survival in a rat model of sepsis via down‐regulation of NF‐κB/JNK: a possible role of P‐glycoprotein. Inflammopharmacology. 2020;28(1):215‐230. [DOI] [PubMed] [Google Scholar]
- 15. Coomes EA, Haghbayan H. Interleukin‐6 in COVID‐ 19: a systematic review and meta‐analysis. medRxiv preprint server. 2020; 2020.03.30.20048058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Capra R, De Rossi N, Mattioli F, et al. Impact of low dose tocilizumab on mortality rate in patients with COVID‐19 related pneumonia. Eur J Intern Med. 2020;76:31‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morena V, Milazzo L, Oreni L, et al. Off‐label use of tocilizumab for the treatment of SARS‐CoV‐2 pneumonia in Milan, Italy. Eur J Intern Med. 2020;76:36‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramaswamy M, Mannam P, Comer R, Sinclair E, McQuaid DB, Schmidt ML. Off‐label real world experience using tocilizumab for patients hospitalized with COVID‐19 disease in a regional. community health system: a case‐control study. medRxiv. 2020; 2020.05.14.20099234. [Google Scholar]
- 20. Wadud N, Ahmed N, Mannu Shergil M, et al. Improved survival outcome in SARs‐CoV‐2 (COVID‐19) acute respiratory distress syndrome patients with tocilizumab administration. medRxiv. 2020; 2020.05.13.20100081. [Google Scholar]
- 21. Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526‐2533. [DOI] [PubMed] [Google Scholar]
- 22. Jones AE, Trzeciak S, Kline JA. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med. 2009;37(5):1649‐1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. La Rosee P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465‐2477. [DOI] [PubMed] [Google Scholar]
- 24. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301‐1308. [DOI] [PubMed] [Google Scholar]
- 25. United States Food & Drug Administration . Expanded Access. 2019. [updated 5/6/2019; accessed 22/4/2020]. Available from: https://www.fda.gov/news‐events/public‐health‐focus/expanded‐access
- 26. Keusch G. McAdam K. Mancher M. Busta E, Integrating Clinical Research into Epidemic Response: The Ebola Experience. Washington, DC: National Academies Press; 2017;26/6/2017. [PubMed] [Google Scholar]
- 27. Bergin C, Browne P, Murray P, et al. Interim Guidance for the use of Tocilizumab in the Management of Patients who have Severe COVID‐19 with Suspected Hyperinflammation [v3. 0]: Health Service Executive; 2020;2020–03‐31. [Google Scholar]
- 28. Michigan Medicine . Inpatient Guidance for Treatment of COVID‐19 in Adults and Children. Lansing, MI: University of Michigan; 2020. 1/4/2020. [Google Scholar]
- 29. Tanaka T, Narazaki M, Kishimoto T. Immunotherapeutic implications of IL‐6 blockade for cytokine storm. Immunotherapy. 2016;8(8):959‐970. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
All authors had full access to all of the data in the study.


