Abstract
Background
Globally, coronavirus disease‐2019 (COVID‐19) is a new, highly contagious, and life‐threatening virus. We aimed to demonstrate how we proceeded with bronchoscopic procedures without published guidelines at the inception of the pandemic period.
Materials and Methods
All bronchoscopic procedures applied from the first case seen in Turkey (11 March‐15 May) were evaluated retrospectively. Patient data on indications, diagnosis, types of procedures, and the results of COVID‐19 tests were recorded.
Results
This study included 126 patients; 36 required interventional bronchoscopic techniques (28.6%), 74 required endobronchial ultrasonography (EBUS; 58.7%), and 16 required flexible fiberoptic bronchoscopy (12.7%). All interventional rigid bronchoscopic techniques were performed for emergent indications: malignant airway obstruction (66.7%), tracheal stenosis (25%), and bronchopleural fistula (8.3%). Malignancy was diagnosed in 59 (79.7%), 12 (50%), and 4 (25%) patients who underwent EBUS, interventional procedures, and fibreoptic bronchoscopy, respectively. All personnel wore personal protective equipment and patients wore a surgical mask, cap, and disposable gown. Of the patients, 31 (24.6%) were tested for COVID‐19 and all the results were negative. COVID‐19 was not detected in any of the patients after a 14‐day follow‐up period.
Conclusion
This study was based on our experiences and demonstrated that EBUS and/or bronchoscopy should not be postponed in patients with known or suspected lung cancer.
Keywords: bronchoscopy, COVID‐19, endobronchial ultrasonography, interventional bronchoscopy, lung cancer
Abbreviations
- APC
argon plasma coagulation
- COVID‐19
coronavirus disease‐2019
- CR
cryo‐recanalization
- EBUS‐TBNA
endobronchial ultrasonography guided‐transbronchial needle aspiration
- MSSA
methicillin‐susceptible Staphylococcus aureus
- MTR
mechanical tumor resection
- PPE
personal protective equipment
- RT‐PCR
reverse transcription‐polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus‐2
- WHO
World Health Organization
1. INTRODUCTION
Coronavirus disease‐2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), is a life‐threatening and highly contagious virus that has resulted in more than (as of 18 May) 300 000 deaths worldwide. 1 COVID‐19 is a member of the coronavirus family and was first reported in Wuhan, China in December 2019. It was declared a global pandemic by the WHO on 11 March 2020. 2 , 3 Since December 2019, COVID‐19 has spread rapidly and, today, there are over four million cases (as of May 18) of infection in nearly 200 countries worldwide. 1
The route of transmission is not yet exactly understood; it is thought to be mainly transmitted through respiratory droplets or by direct contact. However, COVID‐19 has also been detected in nonrespiratory specimens, such as stool, blood, ocular secretions, and semen, but the role of these sites in the transmission is uncertain. 4 , 5 , 6 As of 18 May, as in many countries, the number of daily new diagnosed cases in Turkey is still over 1000. 1 This is due to the ability of the COVID‐19 virus to spread rapidly from human to human through direct contact or respiratory droplets. Especially within 2 m of an infected person, the transmission risk is even higher. 7
Although bronchoscopy is a procedure used for the diagnosis and treatment of various conditions, it is also known as an aerosol‐generating procedure, so it results in a high risk of infection for health care workers during the COVID‐19 pandemic. 8 On the other hand, throughout the pandemic, patients continued to be admitted with symptoms not related to COVID‐19 infection, but as a result of suspected lung cancer instead. In these patients, bronchoscopy or endobronchial ultrasonography (EBUS) played an important role in both diagnosis and treatment. In this study, we reviewed the bronchoscopic procedures performed during the pandemic in an interventional pulmonology unit in Turkey and stated how we proceeded with these bronchoscopic procedures and precautions without any published guidelines on this subject.
2. MATERIALS AND METHODS
This study was designed to be retrospective, cross‐sectional, and descriptive. Patients who were referred to the Interventional Pulmonology Unit of Ataturk Chest Diseases and Thoracic Surgery Training and Research Hospital (of the Health Science University) for further examination with a diagnosis or suspected case of lung cancer were included in this study. The medical files of 126 patients following the first case seen in Turkey (11 March 2020‐15 May 2020; ∼8 weeks) were evaluated retrospectively.
Bronchoscopic procedures were grouped as flexible fiberoptic bronchoscopy (FOB) in an intensive care unit or a standard bronchoscopy room, interventional bronchoscopic techniques via rigid bronchoscopy, and EBUS guided‐transbronchial needle aspiration (EBUS‐TBNA).
All interventional procedures were performed under general intravenously administered anesthesia. Patients were intubated with a rigid Effer‐Dumon bronchoscope (Effer Endoscopy, Marseille, France), and different diameters were preferred according to the type of procedure. Debulking procedures were performed by mechanical tumor resection (MTR) using the tip of the rigid bronchoscope, rigid forceps or argon plasma coagulation (APC)‐assisted MTR (ERBE ICC 200/APC 300 electrosurgical unit, rigid APC probe, 50 cm length, 2.3 mm diameter; ERBE Medizintechnik GmbH, Tübingen, Germany), or cryo‐recanalization (CR; ERBOKRYO CA unit, ERBE rigid cryoprobe 3 mm diameter, 53 cm length; ERBE Medizintechnik GmbH, Tübingen, Germany). A convex EBUS probe (BF‐UC180F; Olympus, Tokyo, Japan) with a dedicated scanner (EU‐ME1; Olympus, Tokyo, Japan) was used to examine the lymph nodes of patients.
SARS‐CoV‐2 reverse transcription‐polymerase chain reaction (RT‐PCR) tests were performed on patients with symptoms suggestive of COVID‐19 and patients from cities with high COVID‐19 positivity. Patient data on gender, age, indications, diagnosis, types of procedures, and RT‐PCR tests were recorded. After the procedure, the patients were monitored for COVID‐19 infection for 2 weeks. The symptoms of the health care workers who performed the procedures were also monitored closely. A high‐level of disinfection was used in line with manufacturer instructions and professional guidelines for the disinfection of reusable bronchoscopes.
Statistical analysis was performed using SPSS 16.0 software (IBM, New York, NY). Descriptive statistics were given as mean ± SD, number, and percentage values.
This study has been approved by both the local ethics committee and the Republic of Turkey Ministry of Health. Informed consent was obtained from all patients.
3. RESULTS
Of the 126 patients enrolled in the study, 100 (79.3%) were male and 26 (20.7%) were female. Bronchoscopic procedures were grouped as FOB in the intensive care unit and standard bronchoscopy room, interventional rigid bronchoscopic techniques, and EBUS‐TBNA. EBUS‐TBNA was performed on 74 patients, interventional procedures on 36 patients and FOB on 16 patients. The patients' mean ages were 60 ± 12 (21‐86), 62 ± 8 (39‐78), and 59 ± 16 (21‐76) years in these groups, respectively.
All procedures were done by a minimum possible number of health care workers, such as one or two bronchoscopists, one member of the medical staff, an anesthesiologist, and a technician. All physicians and ancillary staff were tested for COVID‐19 during hospital health screening and no positivity was detected. Only one ancillary staff was tested again after traveled to another city and resulted as negative but he was also isolated for 14 days then continued to work again together with us.
Indications of procedures and types of interventional techniques performed are shown in Figure 1. Accordingly, 66.7% (n = 24) of patients that underwent interventional procedures had a malignant central airway obstruction and, of these patients, 50% (n = 12) had no diagnosis (Figure 2A‐F). While APC‐assisted MTR was the most frequently used technique (n = 12), CR in addition to APC‐assisted MTR was also used (n = 7). Dilatation with CR (n = 2) and MTR only (n = 2) were the other techniques used. All patients were presented with a diagnosis (Table 1) and treatments were started as soon as possible. A silicone Y‐stent was removed in one patient due to mucostasis, which caused severe dyspnea. Nine patients with postintubation tracheal stenosis presented with severe stridor. All patients were treated by tracheal dilatation and cryotherapy to the stenosis line. Two patients underwent rigid bronchoscopy three times due to bronchopleural fistula. Stents in two patients were deployed (Figure 2I,J), although we had to perform a second procedure on one patient due to the migration of the stent. Before the procedures, 12 patients (33.3%) were tested for COVID‐19, and all results were negative.
Figure 1.
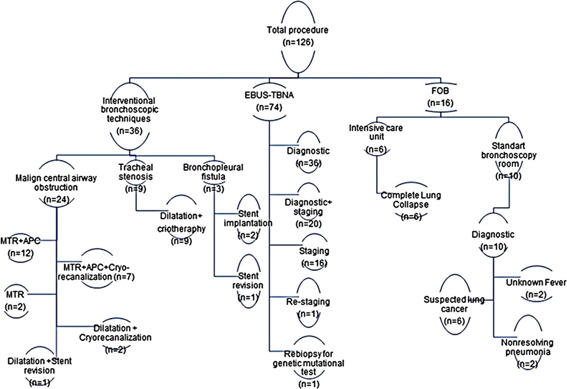
Flow chart of the study
Figure 2.
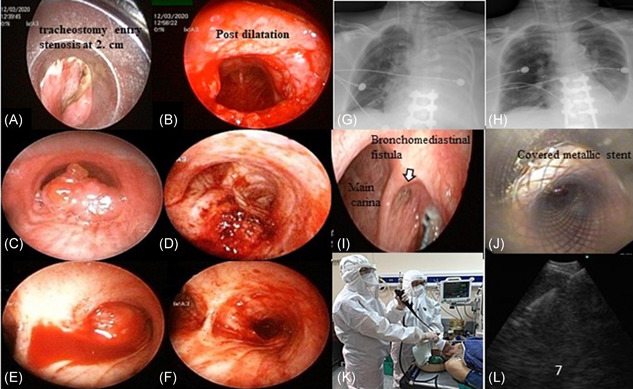
Examples of cases in the study, A‐J. (A and B: pre and postprocedure view of postintubation tracheal stenosis; C and D, pre and postprocedure view of tracheal malign airway obstruction with adenoid cystic carcinoma; E and F, pre and postprocedure view of central (right main bronchus) malign airway obstruction with squamous cell carcinoma; G and H, pre and postprocedure chest X‐ray of left lung complete atelectasis with mucus plaque; I and J, bronchomediastinal fistula, and after covered with metallic stent). K, Protection of health workers and patient's during EBUS‐TBNA L: sonographic view of a sampled subcarinal lymph node. EBUS‐TBNA, endobronchial ultrasonography guided‐transbronchial needle aspiration [Color figure can be viewed at wileyonlinelibrary.com]
Table 1.
Demographic features of patients in interventional procedures, EBUS, and FOB groups
| EBUS‐TBNA | Sex | N (%) | Diagnosis | Patients N (%) | COVID‐19 test (RT‐PCR) | N (%) | City | N (%) | Sampled lymph nodes | N (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Male | 60 (81%) | Lung cancer | Positive | 0 | Ankara | 45 (61%) | Subcarinal (7) | 63 (38.4%) | ||
| Female | 14 (19%) | Adenocarcinoma | 18 (24%) | Negative | 11 (15%) | Other | 29 (39%) | Right lower paratracheal (4R) | 28 (17.1%) | |
| Squamous cell | 12 (16%) | Not tested | 63 (85%) | |||||||
| NOS | 12 (16%) | |||||||||
| Small cell lung cancer | 6 (8%) | |||||||||
| Sarcoidosis | 5 (7%) | Left Lower paratracheal (4L) | 26 (15.9%) | |||||||
| Anthracosis | 5 (7%) | |||||||||
| Other malignancies | 11 (15%) | Left interlobar (11L) | 21 (12.8%) | |||||||
| Reactive lymph node | 3 (4%) | Right interlobar (11R) | 17 (10.4%) | |||||||
| Amyloidosis | 1 (1.5%) | Right hilar (10R) | 5 (3%) | |||||||
| Tuberculosis | 1 (1.5%) | Left hilar (10L) | 2 (1.2%) | |||||||
| Left upper paratracheal (2L) | 2 (1.2%) | |||||||||
| Total | 74 (100%) | Total | 74 (100%) | Total | 74 (100%) | Total | 74 (100%) | Total | 164 (100%) | |
| INT. PROC | ||||||||||
| Male | 30 (83%) | Lung cancer | 23 (64%) | Positive | 0 | Ankara | 23 (64%) | |||
| Female | 6 (17%) | PITS | 9 (25%) | Negative | 12 (33%) | Other | 13 (36%) | |||
| Total | 36 (100%) | Other a | 4 (11%) | Not tested | 24 (67%) | |||||
| FOB | Bronchial washing culture | N (%) | ||||||||
| Male | 10 (62.5%) | Lung Cancer | 3 (19%) | Positive | 0 | Ankara | 11 (69%) | Not taken | 7 (44%) | |
| Female | 6 (37.5%) | Mucoepidermoid Carcinoma | 1 (6%) | Negative | 8 (50%) | Other | 5 (31%) | Culture b (+) | 5 (31%) | |
| Granulation | 1(6%) | Not tested | 8 (50%) | Culture (−) | 4 (25%) | |||||
| Total | 16(100%) | Not Biopsied | 11 (69%) | Total | 16(100%) | Total | 16(100%) | |||
| Total | 16 (100%) |
Abbreviations: EBUS‐TBNA, endobronchial ultrasonography guided‐transbronchial needle aspiration; FOB, fiberoptic bronchoscopy; INT. PROC, interventional procedures; NOS, not otherwise specified; PITS, postintubation tracheal stenosis.
OTHER: Condroid hamartoma, lipomatous hamartoma, adenoid cystic carcinoma, and malignant melanoma metastasis.
Culture: +Methicillin‐susceptible Staphylococcus aureus, Pseudomonas aeruginosa, Acinetobacter baumanii (2), Mycobacterium tuberculosis.
There were 164 lymph nodes sampled in 74 patients under deep sedation (Figure 2K,L). The demographic characteristics are summarized in Table 1. EBUS‐TBNA was mostly performed to diagnose suspected malignancy (n = 36; 49%); however, it was also used for simultaneous diagnosis and staging in 20 patients (27%) and staging in a further 16 patients (22%; Figure 1). In 61% (34/56) of patients who received EBUS‐TBNA, the result was a diagnosis of lung cancer. As a result, of the patients who underwent EBUS, 59 (79.7%) were diagnosed with malignancy and quickly began treatment (Table 1). Before the procedures, 11 patients (15%) were tested for COVID‐19, and all results were negative.
FOB was performed in the standard bronchoscopy room through the transnasal passage under mild sedation on 10 patients with suspected lung cancer (n = 6), nonresolving pneumonia (n = 2), and a fever of unknown origin (n = 2). Of the six patients who underwent FOB for suspected lung cancer, mucoepidermoid carcinoma (n = 1), adenocarcinoma (n = 1), and non‐small–cell lung cancer (n = 2) were diagnosed in four patients; pleural effusion and lymph nodes were sampled in the remaining two patients. Methicillin‐susceptible Staphylococcus aureus and Pseudomonas aeruginosa were obtained from the bronchial washings of two patients performed due to a fever of unknown origin. GeneXpert (Cepheid Inc; Sunnyvale, CA) assay for Mycobacterium tuberculosis was positive in bronchial washing in a nonresolving pneumonia case and specific treatments were started. In addition, FOB was applied through an endotracheal tube in six patients with complete lung collapse who could not be provided with adequate oxygenation with mechanical ventilation. After mucus and fibrin plugs were removed, ventilation was provided in all patients (Figure 2G,H) and Acinetobacter baumannii positivity was also detected in two patients' bronchial washing cultures. Before the procedure, the COVID‐19 PCR test via oropharyngeal/nasopharyngeal swabs were performed in 8/16 patients (50%, six patients of which were in the intensive care unit) and all were found to be negative.
4. DISCUSSION
The first case of COVID‐19 in Turkey was reported on 11 March 2020 9 and an increasing number of infections were quickly reported. The Republic of Turkey Ministry of Health quickly made arrangements in the health system. First, pandemic hospitals were determined in all cities but specific branch hospitals, such as oncology and respiratory units, were excluded; these hospitals continued working routinely, which was extremely crucial for malignant patients with ongoing treatment and patients with suspected cancer. However, COVID‐19 polyclinics were opened in all hospitals across the country and all patients who applied to these outpatient clinics were tested in line with the guidelines: cough, fever, shortness of breath, a history of coming from abroad, or a history of contact with another patient with COVID‐19. 9 About 2 weeks following the first identified COVID‐19 case, the possible case definition was changed, and all those with a cough or shortness of breath and a fever were tested for COVID‐19. 10 Second, an announcement that no patients with COVID‐19 should be directed to appointed nonpandemic hospitals was declared. Thus, the minimization of transmission from patients with COVID‐19 to other patients or health care workers was ensured. Third, health care workers were allowed to rest by applying for rotation. Lastly, guidelines were constantly updated by a scientific committee by closely following the global developments of the pandemic.
COVID‐19 is known to be transmitted through respiratory droplets and direct contact; the risk of transmission is higher within ∼2 m from an infected person; however, the maximum distance is still undetermined. 11 Bronchoscopy is an aerosol‐generating procedure that leads to the pronounced formation of aerosols and thus poses a high risk of infection to proximate health care staff. 8 In addition, nosocomial outbreaks and pseudo‐outbreaks caused by various bacteria or viruses linked to inadequately processed bronchoscopes have been reported in several studies. 7 , 12 The present study site is a training and research hospital for respiratory diseases and thoracic surgery specifically in Ankara, Turkey, and was, therefore, classified as a nonpandemic hospital. Therefore, during the pandemic, patients with symptoms and signs of suspected lung cancer were referred from all over the country and admitted to our clinic for further examination and treatment. At the onset of the pandemic, there were no national or international guidelines determined for bronchoscopic procedures, which presented a significant problem. Although the COVID‐19 Science Committee Guideline was updated on 14 April, there was still no clear recommendation regarding bronchoscopic procedures, 13 so the precautions applied before, during, and after this point in relation to bronchoscopic procedures were determined together with hospital administration and an infection committee. We assigned the precise bronchoscopy indications for both outpatients and the intensive care unit patients and implemented the applications outlined below within the framework of protection measures determined by the Centers for Disease Control and Prevention in the United States. 14
4.1. In the bronchoscopy unit
All procedures were performed in an independent bronchoscopy unit with separate ventilation and high‐efficiency particulate air filters, close to intensive care; we did not have a negative pressure room. Staff were reduced to a minimum throughout the day by creating a core team including a bronchoscopist, bronchoscopy assistants, nurses, and an anesthetist who carried out all the daily interventions. Preprocedure, in the bronchoscopy unit and resting area, a surgical mask was worn by all patients. All personnel in the operating room were dressed in personal protective equipment (PPE), including an N95 mask, eye protection (reusable safety glasses), disposable gloves, an impervious gown, a face shield, and a cap. All procedures were implemented under deep sedation or general anesthesia. For flexible bronchoscopy, a transnasal approach was preferred. For rigid bronchoscopy, conventional closed‐circuit ventilation, and reduction of the aerosol leakage were preferred; jet ventilation was avoided.
4.2. After bronchoscopy
Patients who underwent EBUS‐TBNA and FOB were discharged on the same day and were suggested to follow‐up if they experienced symptoms such as fever, dyspnea, and cough for 14 days postprocedure. When the patients returned to the clinic after 10 days to receive their results, they were asked to disclose any of the aforementioned symptoms. Standard disinfection protocols were followed for the cleaning of bronchoscopes and video monitors, and at least 30 minutes were given for disinfection and ventilation between procedures.
In line with these precautions taken during the first 2 months of the pandemic period, a total of 36 interventional procedures, 74 EBUS‐TBNA, and 16 FOB were performed. Even though Ost DE recommends testing all patients before the procedure, we only tested 31 of 126 (24.6%) patients. 15 Although this was due to the difficulty of accessing tests during the initial pandemic, we decided it was more appropriate to test patients with a higher likelihood of infection based on symptoms, since the false negativity of the test was about 67% in the following days. 16 The first American Association for Bronchology and Interventional Pulmonology statement was published in late March; in the following days, expert opinions on the use of bronchoscopy during the COVID‐19 pandemic were published one after another. 17 , 18 , 19 , 20 , 21 While the use of PPE and bronchoscopy indications were similar between the published reports and our practice, not using negative pressure rooms, not testing all patients, using a reusable bronchoscope and only giving a 30‐minute interval between the procedures are the notable differences between the reports and our practice. We proved that these differences did not cause any problems in the detection of COVID‐19 in either patients and health care workers after the procedure.
Although it has been reported that a brief delay (2‐3 weeks) will not harm cancer diagnosis and staging, 22 how long the COVID‐19 pandemic will continue is still unknown. Bronchoscopy is often required both for diagnosis and staging, and timeliness of bronchoscopic diagnosis impacts every stage of lung cancer care. Also, EBUS for staging and diagnosis as the first test in patients with T1‐3, N1‐3, and M0 disease has been shown to decreases complications, the number of tests required and time to treatment. 23 Moreover, delays in care may lead to missed opportunities in terms of cure or palliation for lung cancer. So, whether it is judicious to delay bronchoscopy or EBUS of patients with known or suspected lung cancer in this unknown process is the question at hand. Based on our experience, the answer to this question is no, EBUS and/or bronchoscopy should not be postponed in patients with known or suspected lung cancer; we determined that COVID‐19 transmission can be prevented by taken sufficient and necessary precautions.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
SYNOPSIS
There is not yet any published experience of bronchoscopic procedures during the coronavirus disease‐2019 (COVID‐19) pandemic in the literature and many countries, especially in Europe and America, are still struggling with the challenging COVID‐19 virus. As a result, the bronchoscopic procedures we performed during the pandemic were very similar to our daily routine practices. We hope this study will shed light on our experiences, both during the pandemic period and as we switch back to routine life.
Ozturk A, Sener MU, Yılmaz A. Bronchoscopic procedures during COVID‐19 pandemic: Experiences in Turkey. J Surg Oncol. 2020;122:1020–1026. 10.1002/jso.26164
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. World Health Organization . Coronavirus disease (COVID‐2019) situation report‐119.
- 2. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019‐nCoV). World Health Organization; 30 January 2020. Archived from the original on 31 January 2020. Accessed January 30, 2020.
- 4. Wang W, Xu Y, Gao R, et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA. 2020;323(18):1843‐1844. 10.1001/jama.2020.3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open. 2020;3(5):e208292. 10.1001/jamanetworkopen.2020.8292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colavita F, Lapa D, Carletti F, et al. SARS‐CoV‐2 isolation from ocular secretions of a patient with COVID‐19 in Italy with prolonged viral RNA detection [published online ahead of print April 17, 2020]. Ann Intern Med. 2020. 10.7326/M20-1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ofstead CL, Hopkins KM, Binnicker MJ, Poland GA. Potential impact of contaminated bronchoscopes on novel coronavirus disease (COVID‐19) patients. Infect Control Hosp Epidemiol. 2020:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tran K, Cimon K, Severn M, Pessoa‐Silva CL, Conly J. Aerosol‐generating procedures and risk of transmission of acute respiratory infections: a systematic review. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2011. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3579388/ [PubMed]
- 9.The Republic of Turkey Ministry of Health Covid‐19 Science Committee Guideline, March 11, 2020; first edition.
- 10.The Republic of Turkey Ministry of Health Covid‐19 Science Committee Guideline, update on March 23, 2020.
- 11. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Galdys AL, Marsh JW, Delgado E, et al. Bronchoscope‐associated clusters of multidrug‐resistant Pseudomonas aeruginosa and carbapenem‐resistant Klebsiella pneumoniae . Infect Control Hosp Epidemiol. 2019;40:40‐46. [DOI] [PubMed] [Google Scholar]
- 13.The Republic of Turkey Ministry of Health, General Directorate of Public Health, COVID‐19 Guideline (SARS‐CoV‐2 Infection), Science Committee Work (April 14, 2020: last update).
- 14. Centers for Disease Control and Prevention . Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease (COVID‐19) in healthcare settings (Updated 2020). https://www.cdc.gov/coronavirus/2019-ncov/infection/control/control/recommendations.html. Accessed March 23, 2020.
- 15. Ost DE. Bronchoscopy in the age of COVID‐19. J Bronchol Interv Pulmonol. 2020;27:160‐162. 10.1097/LBR.0000000000000682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J. Variation in false‐negative rate of reverse transcriptase polymerase chain reaction–based SARS‐CoV‐2 tests by time since exposure [published online ahead of print May 13, 2020]. Ann Intern Med. 2020. 10.7326/M20-1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wahidi MM, Lamb C, Murgu S, et al. American Association for Bronchology and Interventional Pulmonology (AABIP) statement on the use of bronchoscopy and respiratory specimen collection in patients with suspected or confirmed COVID‐19 infection [published online ahead of print March 18, 2020]. J Bronchol Interv Pulmonol. 2020. 10.1097/LBR.0000000000000681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luo F, Darwiche K, Singh S, et al. Performing bronchoscopy in times of the COVID‐19 pandemic: practice statement from an international expert panel [published online ahead of print April 28, 2020]. Respiration. 2020;1:6‐422. 10.1159/000507898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wahidi MM, Shojaee S, Lamb CR, et al. The use of bronchoscopy during the COVID‐19 pandemic: CHEST/AABIP guideline and expert panel report [published online ahead of print May 01, 2020]. Chest. 2020. 10.1016/j.chest.2020.04.036 [DOI] [Google Scholar]
- 20. Lentz RJ, Colt H. Summarizing societal guidelines regarding bronchoscopy during the COVID‐19 pandemic [published online ahead of print April 11, 2020]. Respirology. 2020;25:574‐577. 10.1111/resp.13824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Steinfort DP, Herth FJF, Irving LB, Nguyen PT. Safe performance of diagnostic bronchoscopy/EBUS during the SARS‐CoV‐2 pandemic [published online ahead of print May 13, 2020]. Respirology. 2020;25:703‐708. 10.1111/resp.13843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ost DE, Jim Yeung SC, Tanoue LT, Gould MK. Clinical and organizational factors in the initial evaluation of patients with lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2013;143(5 suppl):e121S‐e141S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ost DE, Niu J, Zhao H, Grosu HB, Giordano SH. Quality gaps and comparative effectiveness in lung cancer staging and diagnosis. Chest. 2019;157:1322‐1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.


