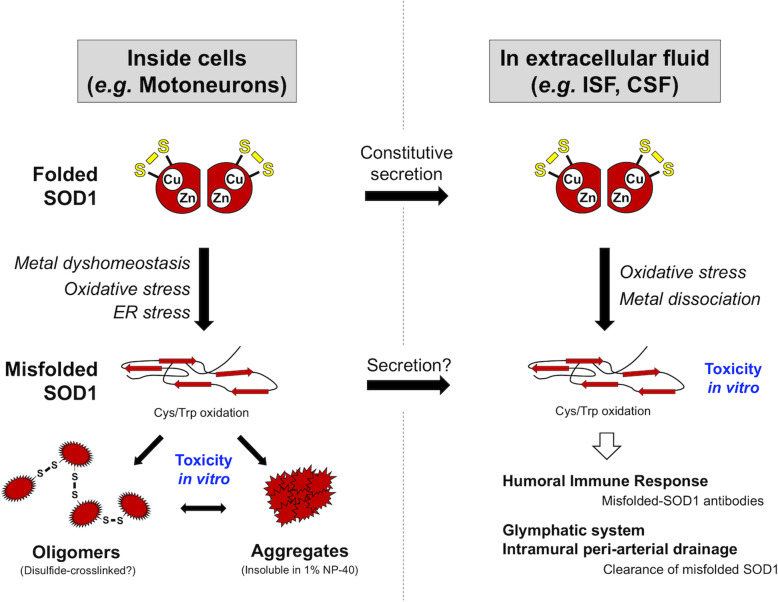
Fig. 1.
Schematic representation on possible changes of wild-type SOD1 in ALS. (Left) A natively folded SOD1 binds copper and zinc ions and forms an intramolecular disulfide bond. Pathological conditions might disrupt intracellular metal homeostasis and augment oxidative stress/ER stress, facilitating the formation of misfolded SOD1 even without any disease-causing mutations. Disulfide-crosslinked oligomers and insoluble aggregates of wild-type SOD1 have been detected in spinal cords of sporadic ALS. (Right) SOD1 has been known to constitutively secreted to extracellular fluid such as ISF and CSF, and recently, toxic wild-type SOD1 in abnormally misfolded conformations was detected in CSF of sporadic ALS. Misfolded SOD1 appears to be cleared by humoral immune response and/or glymphatic/intramural peri-arterial drainage systems, and their failure might contribute to the disease.