Figure 2.
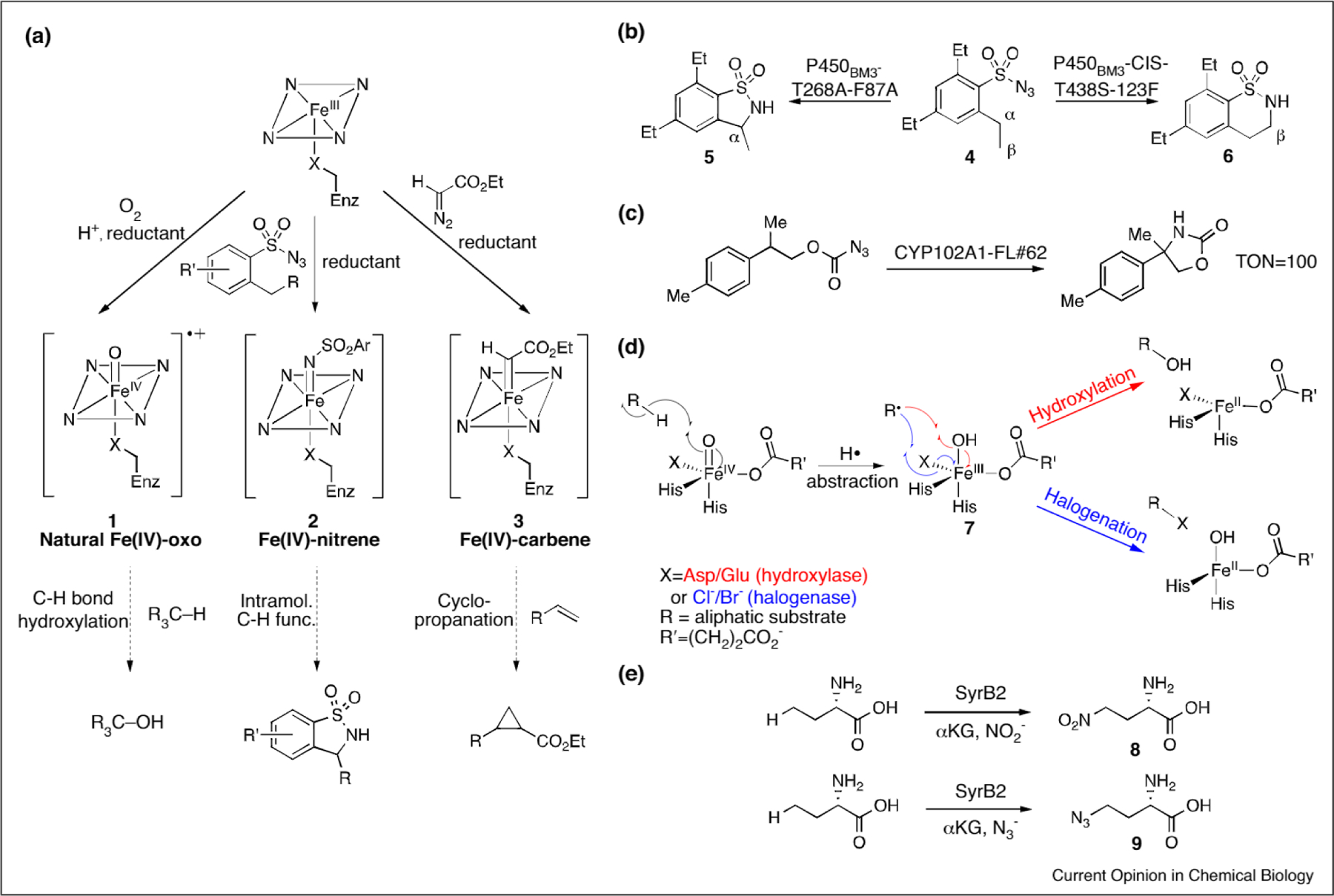
(a) Native and non-native reactions catalyze by heme-containing proteins, (b) P450-catalyzed nitrene insertion into the α (left) or β (right) C–H bond of a sulfonylazide substrate, (c) P450-catalyzed nitrene insertion into the benzylic position of a carbonazidate substrate, (d) Divergent radical rebound mechanism by αKG-dependent hydroxylases (red) and halogenases (blue), (e) Nitration (top) and azidation (bottom) catalyzed by the repurposed FeII- and αKG-dependent halogenase SyrB2.
