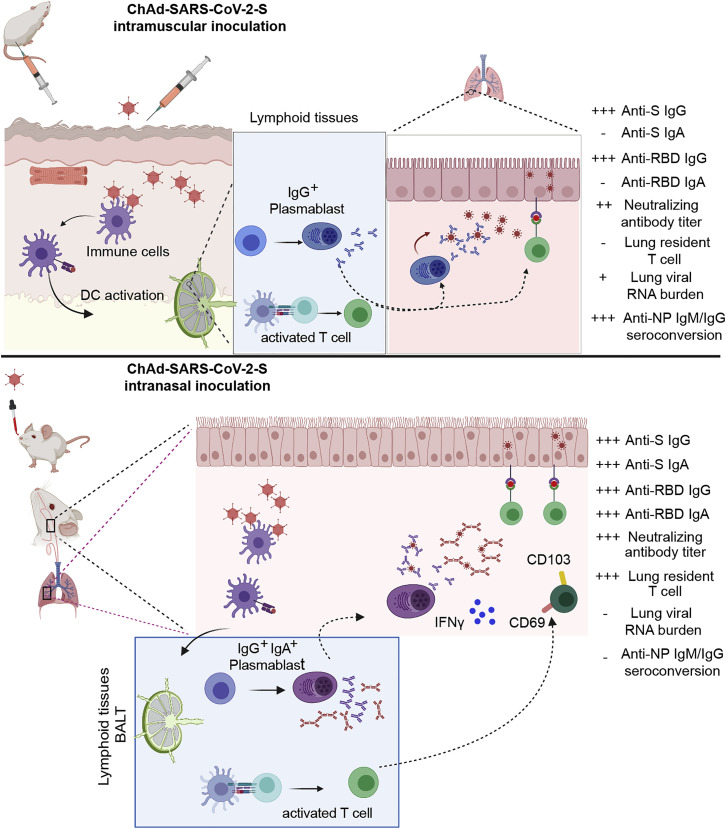
Figure 7.
Comparison of Immunogenicity of Single-Dose Administration of ChAd-SARS-CoV-2-S by Intramuscular and Intranasal Delivery Routes
(Upper panel) After intramuscular inoculation, ChAd-SARS-CoV-2 vaccine transduces antigen-presenting cells (APCs) at the site of injection in muscle tissues. APCs migrate to lymphoid tissues where antigen-specific CD8+T cells become activated, proliferate, and produce IFNγ and granzyme B. Antigen-specific B cells proliferate, some of which become plasmablasts and plasma cells that secrete anti-S IgG. After SARS-CoV-2 challenge, activated CD8+ T cells migrate to the lungs to control infection and anti-S IgG neutralizes virus particles. Intramuscular vaccination protects against lower, but not upper, airway infection and does not efficiently induce mucosal immunity. (Lower panel) After intranasal inoculation, ChAd-SARS-CoV-2 transduces APCs in the upper respiratory tract. APCs then migrate to bronchial- or mucosal-associated lymphoid tissues to present antigens to lymphocytes, including B and T cells. After SARS-CoV-2 challenge, activated CD8+ T cells migrate to the lungs, secrete cytokines, and attack virus-infected cells. Some CD8+ T cells likely adopt a tissue-resident memory phenotype (CD103+ CD69+), enabling them to reside in the lung (or upper airway) and respond more rapidly after re-encountering cognate antigen (SARS-CoV-2 S peptides). The activated B cell becomes responsive after intranasal vaccination produces cells that secrete anti-SARS-CoV-2-S IgG or IgA, the latter of which neutralizes virus within the upper and lower respiratory tracts. The mucosal immunity generated by intranasal inoculation of ChAd-SARS-CoV-2 likely controls infection at the point of initiation in the upper respiratory tract.