Fig. 4. Charge swap experiments do not support the pairing of CXCR4 residues Glu32(NT) and Asp181(ECL2) with CXCL12 Arg8 and Arg12, respectively.
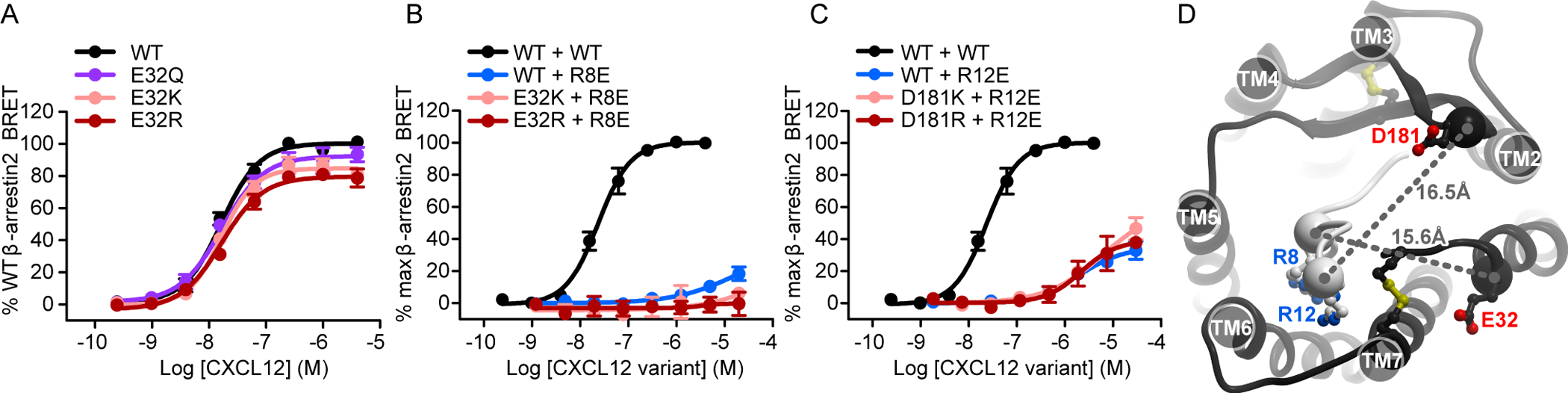
(A) β-arrestin2 association BRET ratio data for a series of CXCR4 Glu32(NT) mutants (E32Q/K/R) after stimulation with the indicated concentrations of CXCL12 for 20 min. (B) β-arrestin2 association BRET ratio data for the indicated concentrations of CXCL12 obtained by stimulating WT CXCR4 with WT CXCL12 or by stimulating WT CXCR4, CXCR4(E32K), or CXCR4(E32R) with CXCL12(R8E). (C) β-arrestin2 association BRET ratio data for the indicated concentrations of CXCL12 obtained by stimulating WT CXCR4 with WT CXCL12 or by stimulating WT CXCR4, CXCR4(D181K), or CXCR4(D181R) with CXCL12(R12E). Data are means ± SEM of at least three independent experiments, each performed in duplicate. Data were normalized to the Emax of WT CXCR4 tested in the same experiments. The same pooled datasets for WT CXCR4 + WT CXCL12, WT CXCR4 + CXCL12(R8E), and WT CXCR4 + CXCL12(R12E) are shown in (B) and (C) as were shown in Fig. 2, E to H. (D) The relative location of the two control residue pairs, CXCR4 Asp181(ECL2) and CXCL12 Arg12, and CXCR4 Glu32(NT) and CXCL12 Arg8, in the model of the CXCR4-CXCL12 complex.
