Figure 2. Dm BicD-CTD/F684I assumes a conformation with homotypic coiled-coil registry.
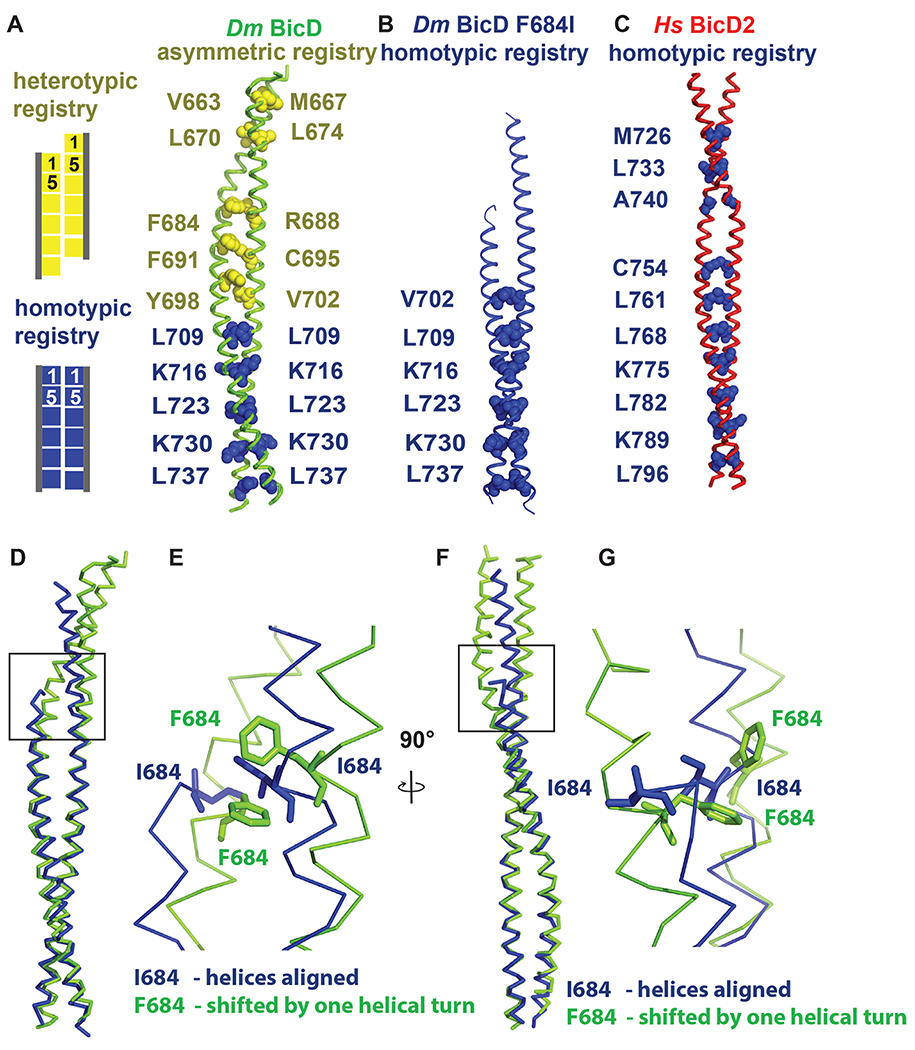
(A) The structure of Dm BicD-CTD wild type (PDB ID 4BL6) 9 which has an asymmetric coiled-coil registry is shown in cartoon representation next to a schematic illustrating coiled-coil registries (left panel). Knob residues in the “a” position of the heptad repeat are shown in spheres representation (heterotypic registry yellow, homotypic registry dark blue). (B) Structure of Dm BicD-CTD/F684I, which has a homotypic registry. (C) Structure of Hs BicD2-CTD (PDB ID 6OFP) 30, which has a homotypic registry. (D-G) Least squares superimposed structures of the Dm BicD-CTD wild type (green) and the F684I mutant (dark blue) are shown as C-α traces, and are rotated by 90° in (D, F). (E, G) Close-up of the boxed area in (D, F). Residues F684 and I684 are shown in stick representation. Note that in the structure of the mutant, the I684 residues from both chains of the dimer are aligned at the same height, consistent with a homotypic registry, while in the wild-type structure, the F684 residues from both monomers are vertically shifted by one helical turn respective to each other, consistent with a heterotypic registry. See Figures S1 and S2.
