Fig. 1. Hypothetical framework for the development of hepatic steatosis in Wilson disease.
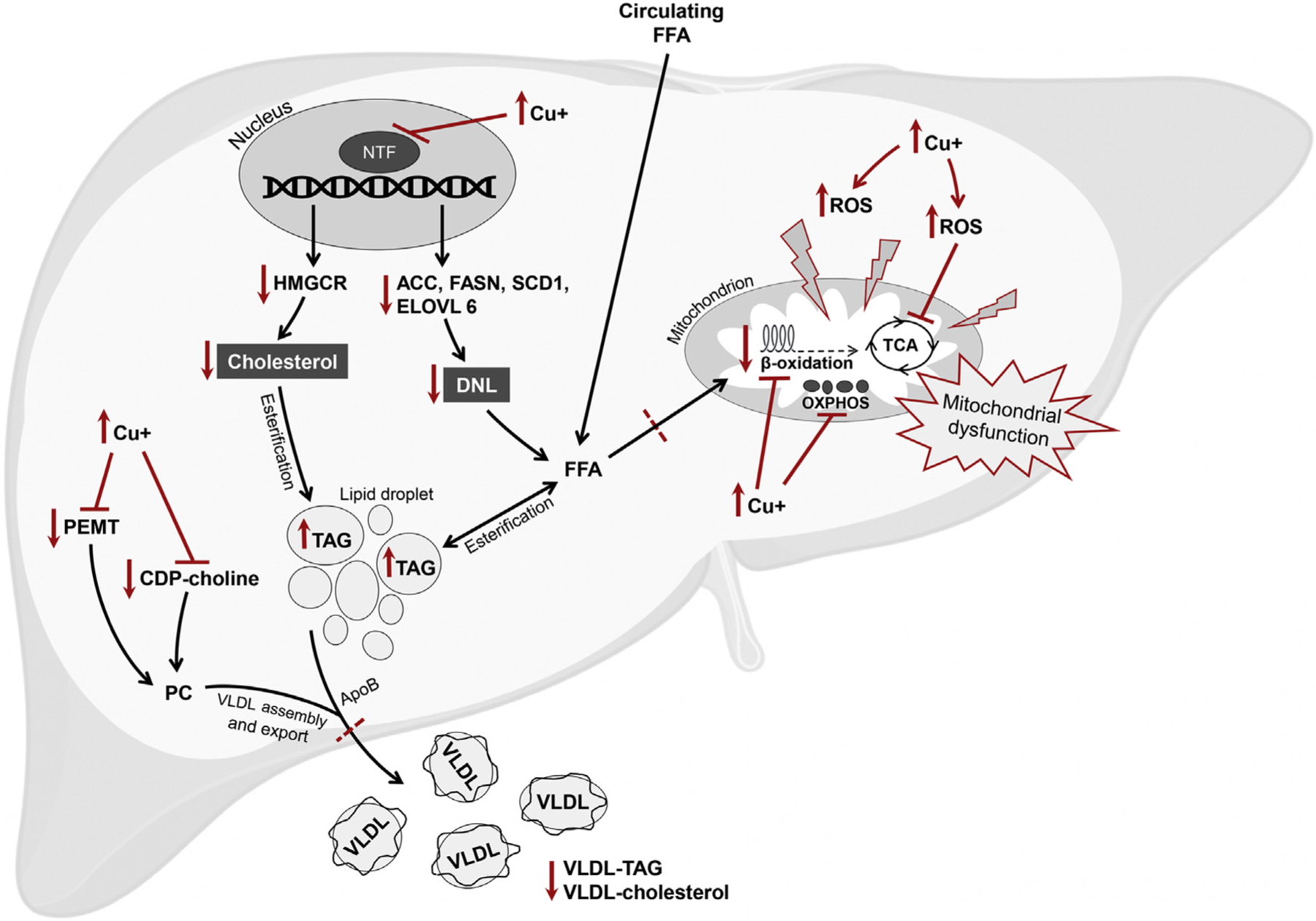
Copper overload induces the production of ROS which interacts with subcellular protein structures, including the mitochondria, to cause structural and functional mitochondrial alteration and bioenergetic defects. Copper may also directly impair enzymes involved in the TCA cycle, fatty acid β-oxidation, and OXPHOS chain. Copper accumulation impairs the function of multiple nuclear transcription factors including retinoid X receptor, liver X receptor, carbohydrate-responsive element-binding protein, and sterol regulatory element-binding protein 1c. The transcript and protein levels of genes involved in cholesterol synthesis and lipogenesis are down-regulated, including HMGCR, ACC, FASN, SCD1, and ELOVL6. The impairment of PC synthesis pathways results in impaired assembly and/or export of VLDL. Together, the uptake of FFA and subsequent esterification into TAG, combined with impaired utilization of FFA and impaired assembly and/or export of VLDL, result in the net effect of hepatic lipid accumulation, or steatosis. ACC, acetyl-CoA carboxylase; ApoB, apolipoprotein B; CDP-choline, cytidine-diphosphate-choline; Cu+, free copper; DNL, de novo lipogenesis; ELOVL6, elongation of very long chain fatty acids protein 6; FASN, fatty acid synthase; FFA, free fatty acids; HMGCR, 3-hydroxy-3-methyl-glutaryl-CoA reductase; NTF, nuclear transcription factor; OXPHOS, oxidative phosphorylation; PC, phosphatidylcholine; PEMT, phosphatidylethanolamine methyltransferase; ROS, reactive oxygen species; SCD1: stearoyl-CoA desaturase; TAG, triglycerides; TCA, tricarboxylic acid; VLDL, very low-density lipoproteins.
