Fig. 2. Example mutations from ChABC-37-SH3.
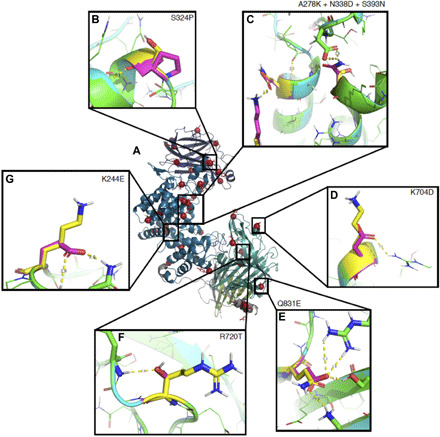
(A) 1HN0 colored by domain as Fig. 1, with residues mutated in ChABC-37-SH3 in magenta. In boxes, native residues are shown in yellow, and mutant residues are shown in magenta. (B) Introducing proline reduces conformational entropy. (C) Three mutations coordinately stabilize the helix termini while maintaining the wild-type helix capping motif. (D and E) Introducing charge-charge interactions increases resistance to aggregation. (F and G) Introducing polar H-bonds stabilizes loops.
