Abstract
Background and Aims: Evaluation of significant liver fibrosis is important for treatment decision and treatment response evaluation in patients with chronic hepatitis B. Since liver biopsy is invasive and transient elastography (TE) has limited availability, various non-invasive blood parameters need evaluation for their capabilities for detection of significant fibrosis.
Methods: In this retrospective study, records of patients who had undergone liver biopsy for treatment-naïve chronic hepatitis B were evaluated to obtain various non-invasive blood parameters (aspartate aminotransferase-to-platelet ratio index [referred to as APRI], Fibrosis-4 score [referred to as FIB-4], gamma-glutamyl transpeptidase-to-platelet ratio [referred to as GPR], and gamma-glutamyl transpeptidase-to-albumin ratio [referred to as GAR]), in addition to TE, to assess significant liver fibrosis and compare these to fibrosis stage in liver biopsy.
Results: A total of 113 patients were included in the study (median age 33 [interquartile range: 11-82 years], 74% males). Most (75%) patients were HBeAg-negative. The liver biopsy revealed significant fibrosis (Ishak ≥3) in 13% of the patients and nil or mild fibrosis (Ishak <3) in 87% of the patients. TE findings were available for 85 patients, APRI and FIB-4 for 95 patients, GPR for 79 patients, and GAR for 78 patients. The median values of all the parameters were significantly higher in patients with significant fibrosis, as compared to patients with non-significant fibrosis, and all the blood parameters as well as TE were able to identify patients with significant fibrosis significantly well (p<0.05). All non-invasive parameters had low positive predictive value but negative predictive value above 92%. Compared to TE, all the non-invasive blood parameters had similar area under the curve for detecting significant fibrosis, with excellent negative predictive value (≥93%).
Conclusions: Non-invasive blood parameters (APRI, FIB-4, GPR, and GAR) with negative predictive values above 93% are excellent parameters for ruling-out significant fibrosis in patients with chronic hepatitis B. These can be used at bedside in place of TE.
Keywords: Hepatitis B, Liver fibrosis, Cirrhosis, Transient elastography, APRI, FIB-4, GPR, GAR
Introduction
Of approximately 2 billion people who have been infected with hepatitis B virus (HBV) worldwide, more than 248 million (5–7% of the world’s population) suffer from chronic HBV infection (CHB) and about 1 million of these die per year.1 India has over 40 million HBV carriers, accounting for 10-15% of the total HBV carriers in the world.2
HBV has a complex natural history, and the interaction between viral proteins and the immune system leads to a cycle of hepatocyte damage and tissue repair.3 This repair leads to progressive liver fibrosis over time, which can be rapid, slow, or sporadic depending on disease state and the degree of active liver inflammation and injury. The assessment of liver fibrosis is vital to disease prognostication and to determining the need for treatment as well as the response to therapy. Studies in Asia and the USA have revealed that 20% to 30% of HBV carriers with persistently normal alanine aminotransferase (ALT) levels and HBV DNA levels >10000 copies/mL have grade ≥2 inflammation and stage ≥2 fibrosis on liver biopsy.4,5 A fair proportion of patients with CHB infection with normal ALT have HBV DNA ≥5 log copies/mL and significant histologic fibrosis.5 At present, the gold standard for assessment of liver fibrosis is liver histology using the Ishak6 or METAVIR7 systems. However, liver biopsy is prone to sampling error and substantial intra- and inter-observer variability, leading to over- or under-staging of fibrosis;8 in addition, the procedure also has significant morbidity, including infections, major bleeding, and ascites leakage, and can lead to mortality.9 Consequently, there is a need for non-invasive methods to accurately diagnose the presence of liver fibrosis and cirrhosis, especially while making a decision to start antiviral therapy.
Transient elastography (TE) has been shown to be an excellent non-invasive modality for assessment of fibrosis;10,11 however, it has limited availability, especially in resource-poor countries. So various non-invasive blood parameters need evaluation to find the most useful parameter for ruling out significant fibrosis.
A number of non-invasive models containing serum markers, such as serum aspartate aminotransferase (AST) to platelet ratio index (APRI),12–14 Fibrosis 4 score (FIB-4),15 gamma-glutamyl transferase (GGT)-to-platelet ratio (GPR),16 and GGT-to-albumin ratio (GAR)17 have been described in the literature. Among these markers, the FIB-4 and APRI12,18 are widely used to assess patients with chronic hepatitis but their value for assessing patients who are chronically infected with HBV remains controversial.19–22 Recently, GPR showed better performance than FIB-4 and APRI in detecting liver fibrosis in CHB West African patients; however, this was not true for French populations.23
There has been no published data from India evaluating the performance of these non-invasive blood parameters for ruling out significant fibrosis in patients with CHB. The aim of the present study was to evaluate and find out the most useful non-invasive blood parameter for ruling out significant fibrosis in CHB and to compare it with TE.
Methods
Patients
This was a retrospective study conducted in the Institute of Liver, Gastroenterology & Panceatico-Biliary Sciences of Sir Ganga Ram Hospital, New Delhi, India. Records of patients with CHB who had undergone liver biopsy between February 2009 and May 2017 were analyzed. The study included consecutive patients who fit the following inclusion criteria: treatment-naïve CHB; age between 10 and 70 years; and had undergone pre-treatment liver biopsy. The following patients were excluded from the study: with co-infection with hepatitis C virus, hepatitis A virus, hepatitis E virus or human immunodeficiency virus; with significant cardiac and/or pulmonary co-morbidities; renal dysfunction (serum creatinine >1.5 mg/dL); grade 3-4 hepatic encephalopathy; with hepatocellular carcinoma or other malignancies; with acute-on-chronic liver failure; or with acute flare of hepatitis (serum bilirubin >4 mg/dL, AST or ALT >300 U/L).
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. The study being a retrospective analysis of data did not require Ethics Committee approval. Also, the retrospective analysis of data, without revealing any patient’s identity, precluded requirement of informed consent from patients.
Liver biopsy
Liver tissue (1.5-2 cm) was obtained by percutaneous or transjugular biopsy by the Gastroenterologist and sent to the Histopathology Department, where it was stained with hematoxylin and eosin. Fibrosis staging was done according to the modified Ishak grading system.6 Significant fibrosis was defined as Grade III or more by modified Ishak grading.
Liver stiffness measurement by TE
Liver stiffness measurement was performed using a FibroScan® device (Echosens, Paris, France), in accordance with the manufacturer’s recommendations. Measurements were made on the right lobe of the liver through intercostal spaces, with the patient lying in a supine position with the right arm in maximal abduction. The tip of the transducer probe was covered with coupling gel and placed on the skin between the rib bones at the level of the right lobe of the liver. When the target area was located, the operator pressed the probe button to commence the measurements. The measurement depth was between 25 mm and 65 mm. Ten successful measurements were performed on each patient. The results were expressed in kPa. The median value was considered as the liver stiffness. Interquartile range/median <30% and success rate >60% were considered as good quality criteria for TE. Patients with significant ascites underwent large volume paracentesis before liver stiffness measurement. All liver stiffness measurements were performed by a single operator.
Laboratory tests
All blood samples were obtained within 1 day of liver biopsy. Blood biochemical parameters included bilirubin, ALT, AST, GGT, albumin, prothrombin time, and platelets. Virological parameters included HBV serological markers and HBV DNA. Non-invasive blood parameters were calculated as per the recommended formulae:12,15–17
APRI = (AST/ [upper limit of normal]/platelet [109/L]) X 100
FIB-4 = (age [year] X AST [U/L]) / {(platelet [109/L]) X (ALT [U/L]) 1/2}
GPR = (GGT/upper limit of normal) X 100/platelet
GAR = GGT/albumin
Statistical analysis
Quantitative data were expressed as median (interquartile range) and compared using the Mann-Whitney U test or Wilcoxon signed-rank test. Qualitative data were expressed as number (%) and compared using Fisher’s exact test or Pearson’s chi-square test. A p value of <0.05 was considered significant. Statistical analysis was conducted using the SPSS 17.0 statistical package (SPSS Inc., Chicago, IL, USA).
Results
Patients
A total of 129 patients were enrolled in the study; however, 16 patients were excluded due to following reasons: co-infection with human immunodeficiency virus (n=1); renal dysfunction (serum creatinine >1.5 mg/dL) (n=3); acute-on-chronic liver failure (n=6); and acute flare of hepatitis (n=6). Hence, the remaining 113 patients were included in the study.
The demographic and biochemical parameters of the included patients is given in Table 1. The median age was 33 (interquartile range of 14) years, and 77% were males. The median HBV DNA was 2×103 (interquartile range of 1×105) IU/dL, and 25% of the patients were positive for hepatitis B e antigen. According to the modified Ishak grading system, 98 (87%) had non-significant fibrosis (Ishak stage <3), while 15 (13%) of patients had significant fibrosis (Ishak stage ≥3). Values of platelet count, GGT, albumin and proportion of patients with hepatitis B e antigen positivity were significantly different between patients with non-significant and significant fibrosis (Table 1).
Table 1. Demographic and biochemical parameters of the study population.
| Parameters | All patients, n=113 | Patients with Ishak <3, n=98 | Patients with Ishak ≥3, n=15 | p value |
| Age in years | 33 (14) | 32 (12) | 45 (24) | 0.138 |
|
Sex Males Females |
87 (77%) 26 (23%) |
74 (76%) 24 (24%) |
13 (87%) 2 (13%) |
0.514 |
| Hemoglobin in g/L | 14.3 (2.1) | 14.3 (2.2) | 14.0 (3.7) | 0.637 |
| White blood cells as ×103/L | 7.0 (3.3) | 7.0 (3.1) | 5.9 (6.0) | 0.992 |
| Platelets as ×106/L | 184 (92) | 187 (96) | 160 (94) | 0.013 |
| Creatinine in mg/dL | 0.8 (0.3) | 0.8 (0.3) | 0.8 (0.5) | 0.159 |
| Bilirubin in mg/dL | 0.7 (0.4) | 0.7 (0.4) | 0.7 (0.4) | 0.615 |
| Aspartate aminotransferase in U/L | 32 (22) | 32 (19) | 69 (114) | 0.080 |
| Alanine aminotransferase in U/L | 38 (40) | 36 (29) | 85 (144) | 0.064 |
| Gamma-glutamyl transpeptidase in U/L | 21 (23) | 20 (20) | 41 (46) | 0.019 |
| Serum alkaline phosphatase in U/L | 90 (45) | 88 (44) | 98 (48) | 0.089 |
| Albumin in g/L | 4.3 (0.5) | 4.3 (0.6) | 4.2 (1.3) | 0.041 |
| International normalized ratio | 1.1 (0.2) | 1.1 (0.1) | 1.1 (0.2) | 0.178 |
| HBV DNA in IU/dL | 2×103 (1×105) | 2×103 (5×104) | 2×104 (5×107) | 0.512 |
| Hepatitis B e antigen-positive | 25% | 17% | 53% | 0.006 |
|
Ishak fibrosis stage 0 1 2 3 4 5 6 |
69 (61%) 25 (22%) 4 (4%) 4 (4%) 2 (2%) 5 (4%) 4 (4%) |
69 (70%) 25 (26%) 4 (4%) |
4 (27%) 2 (13%) 5 (33%) 4 (27%) |
- |
All values are median (interquartile range) or number (%).
Performance of TE in detecting significant fibrosis
TE findings were available for 85 patients, APRI and FIB-4 for 95 patients, GPR for 79 patients, and GAR for 78 patients. All of the five parameters were available for 60 patients. Table 2 shows the comparison of median values of TE, APRI, FIB-4, GPR, and GAR in patients with non-significant fibrosis to those with significant fibrosis. The median values of all the parameters were significantly higher in patients with significant fibrosis, as compared to patients with non-significant fibrosis.
Table 2. Comparison of non-invasive tests between patients with and without significant fibrosis.
| Patients with Ishak <3, n=98 | Patients with Ishak ≥3, n=15 | p value | |
| Transient elastography, n=85 | 5.4 (2.8) | 12.0 (12.6) | 0.004 |
| APRI, n=95 | 0.45 (0.35) | 0.79 (2.34) | 0.013 |
| FIB-4, n=95 | 0.94 (0.68) | 1.92 (3.00) | 0.003 |
| GPR, n=79 | 0.24 (0.22) | 0.46 (1.03) | 0.009 |
| GAR, n=78 | 4.87 (4.64) | 17.98 (21.44) | 0.013 |
All values are median (IQR).
Abbreviations: APRI, aspartate aminotransferase-to-platelet ratio index; FIB-4, Fibrosis-4; GAR, gamma-glutamyl transpeptidase-to-albumin ratio; GPR, gamma-glutamyl transpeptidase-to-platelet ratio.
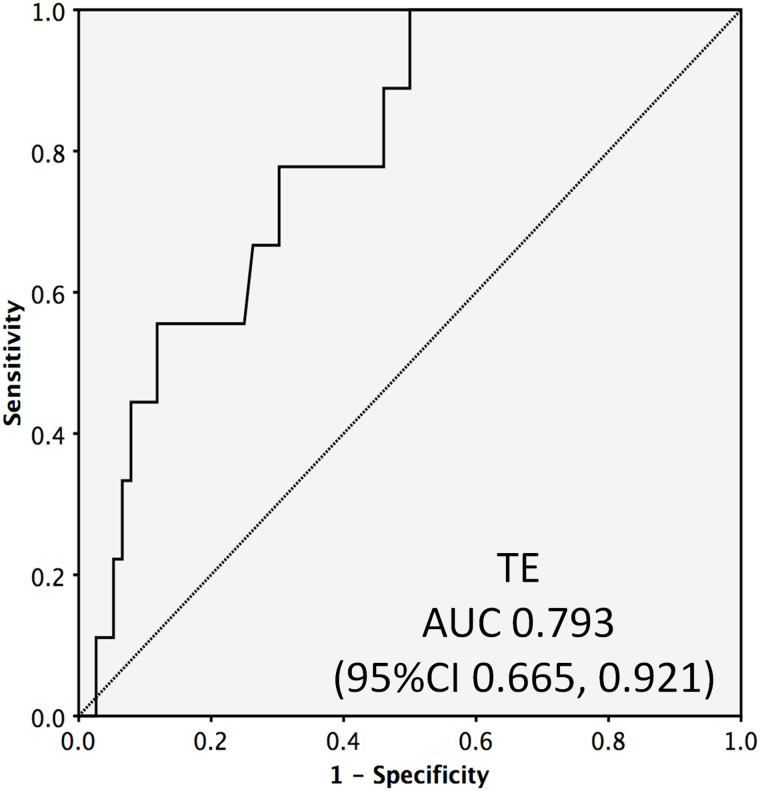
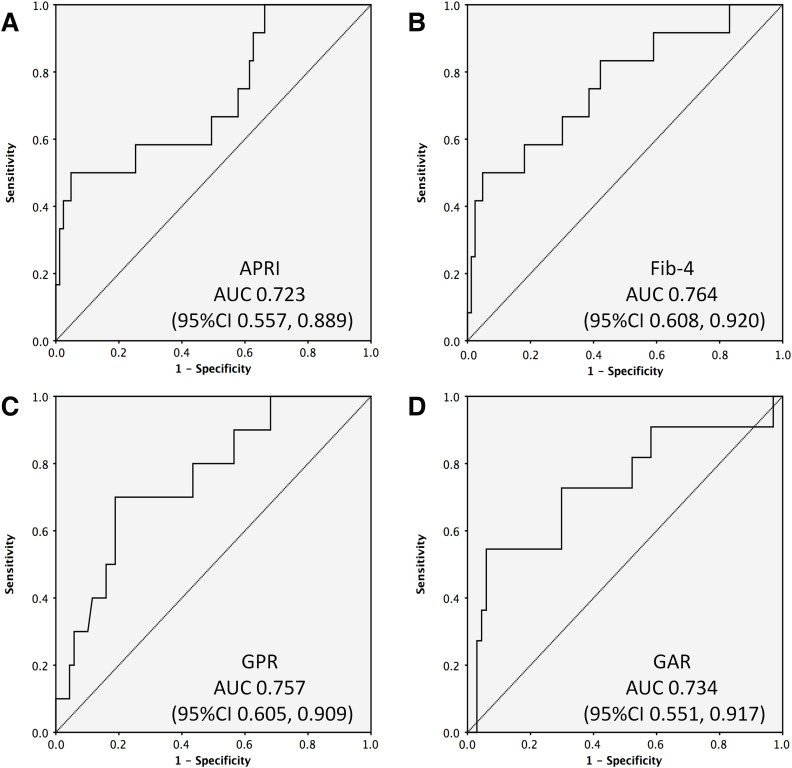
TE had an area under the curve of 0.793 (95% confidence interval of 0.665, 0.921) in the receiver operating characteristic curve for detecting significant fibrosis (Fig. 1). The area under the curve values of APRI, FIB-4, GPR and GAR ranged between 0.723 and 0.764 (Fig. 2), and these values were not significantly different from the area under the curve of TE. Thus APRI, FIB-4, GPR, or GAR can be used in place of TE with similar accuracy.
Fig. 1. Receiver operating characteristic curve for TE for detecting significant fibrosis.
Abbreviations: AUC, area under the curve; CI, confidence interval; TE, transient elastography.
Fig. 2. Receiver operating characteristic curves for detecting significant fibrosis. (A) APRI, (B) FIB-4, (C) GPR, and (D) GAR.
Abbreviations: APRI, aspartate aminotransferase-to-platelet ratio index; FIB-4, Fibrosis-4; GAR, gamma-glutamyl transpeptidase-to-albumin ratio; GPR, gamma-glutamyl transpeptidase-to-platelet ratio.
Table 3 shows the best cut-off values along with positive predictive values (PPVs) and negative predictive values (NPVs) for all the non-invasive parameters for detecting significant fibrosis. All parameters had NPV above 93%. TE had the highest NPV (100%) at a cut-off of <5.35 kPa. Among the blood parameters, GPR had highest NPV (95%) at a cut-off of <0.444. The PPV of all the parameters were low; thus, all these non-invasive tests can be best utilized for ruling out significant fibrosis, rather than ruling in.
Table 3. Performance of transient elastography and non-invasive blood parameters in detecting significant liver fibrosis.
| Parameter | Formula | n | AUROC | 95%CI | p value | Best cut-off | PPV | NPV |
| Transient elastography | - | 85 | 0.793 | 0.665, 0.921 | 0.004 | 5.35 | 19% | 100% |
| APRI | 95 | 0.723 | 0.557, 0.889 | 0.013 | 0.935 | 60% | 93% | |
| FIB-4 | 95 | 0.764 | 0.608, 0.920 | 0.003 | 2.324 | 60% | 93% | |
| GPR | 79 | 0.757 | 0.605, 0.909 | 0.009 | 0.444 | 35% | 95% | |
| GAR | 78 | 0.734 | 0.551, 0.917 | 0.013 | 16.918 | 60% | 93% |
Abbreviations: APRI, aspartate aminotransferase-to-platelet ratio index; FIB-4, Fibrosis-4; GAR, gamma-glutamyl transpeptidase-to-albumin ratio; GPR, gamma-glutamyl transpeptidase-to-platelet ratio; NPV, negative predictive value; Plt, platelet; PPV, positive predictive value; ULN, upper limit of normal.
Table 4 shows a sub-group analysis of only those patients which had data on all the 5 non-invasive parameters. There were a total of 60 patients, and the AUC of all the parameters was still above 0.690.
Table 4. Subgroup analysis of those patients which had data on all the five non-invasive parameters.
| Parameter | N | AUROC | 95% CI |
| TE | 60 | 0.786 | 0.624, 0.948 |
| APRI | 60 | 0.692 | 0.469, 0.916 |
| FIB-4 | 60 | 0.757 | 0.577, 0.938 |
| GPR | 60 | 0.779 | 0.584, 0.974 |
| GAR | 60 | 0.690 | 0.427, 0.953 |
Abbreviations: APRI, aspartate aminotransferase-to-platelet ratio index; AUROC, area under the receiver operating characteristic; CI, confidence interval; FIB-4, Fibrosis-4; GAR, gamma-glutamyl transpeptidase-to-albumin ratio; GPR, gamma-glutamyl transpeptidase-to-platelet ratio; TE, transient elastography.
Discussion
In this study, we compared the diagnostic value of non-invasive blood parameters (APRI, FIB-4, GPR, and GAR) for assessing liver fibrosis in a patients with CHB and found that these blood parameters have NPVs above 93% and are excellent parameters for ruling-out significant fibrosis. These data indicate that these parameters can be used at bedside in place of TE, especially if the latter is not available.
Assessment of significant fibrosis is an important step for decision-making of antiviral treatment in chronically HBV-infected patients.24 The Indian National Asssociation for the Study of the Liver (INASL) guidelines recommend that in patients with hepatitis B e antigen-negative states, if ALT is <80 U/L (i.e. <2×upper limit of normal), HBV DNA is 2,000-20,000 IU/mL, and if non-invasive or invasive assessment of liver fibrosis does not show significant fibrosis, antiviral treatment need not be started and these patients may be kept under observation.2 Similarly, in patients with hepatitis B e antigen-positive state, if ALT is 40-80 U/L, HBV DNA is >20,000 IU/mL, and if non-invasive or invasive assessment of liver fibrosis does not show significant fibrosis, antiviral treatment need not be started as these patients are considered to be in the immune-tolerant phase.2
In the past (over one decade), TE has gained importance as one of the best non-invasive tests to assess liver fibrosis. In our study, TE had the best area under the receiver operating characteristic curve (0.793) compared to the blood parameters. In addition, our cut-off of 5.35 kPa for TE for significant fibrosis was similar to that in a previous French study on 1307 patients which gave a cut-off of 5.2 kPa25 and another study from India which gave a cut-off of 6 kPa.26 We found TE to have the best NPV of 100% when using this cut-off. However, TE has many disadvantages. It is not universally available, especially in resource-poor settings; its applicability is approximately 80%, which is lower than that of serum biomarkers, especially when used in the presence of ascites, obesity, and limited operator experience. It can also lead to false positive values in the case of acute hepatitis, extra-hepatic cholestasis and liver congestion. Finally, it is unable to discriminate between intermediate stages of fibrosis, it requires a dedicated device, and it does not allow for a region of interest to be chosen.27 In contrast, non-invasive serum biomarkers have many advantages: They do not require extra cost and are widely available, can be assessed both in in-patient and out-patient settings, have good reproducibility and high applicability, and most are well validated.27 However, with the multitude of blood parameters, with varying sensitivities and specificities, the best parameter for detection of or for ruling-out significant fibrosis needed evaluation. Hence, in this study, we included commonly used parameters which are available even in most resource-poor settings.
We found that the NPVs of all non-invasive blood parameters were nearly similar and ≥93%. So, all these parameters were found to have similar and excellent performance in ruling-out significant fibrosis in CHB patients (in comparison to liver biopsy). The best cut-off values of GPR, APRI, FIB-4 and GAR, especially for ruling-out of significant fibrosis, were 0.935, 2.324, 0.444 and 17.848. However, the ruling-in performance of these parameters was low, with PPVs of GPR, APRI, FIB-4 and GAR at 28%, 33%, 37% and 35% respectively. GPR was found to have slight superiority because of the highest NPV of 95%, while the NPVs of APRI, FIB-4 and GAR were 93%, 93% and 92% respectively.
APRI is the oldest and probably the most widely used non-invasive parameter to assess liver fibrosis,12,22,28 and even portal hypertension.13,14 In our study, we found the area under the receiver operating characteristic curve of APRI for significant fibrosis was 0.723, and 0.935 was the best cut-off. Our results are similar to a meta-analysis of 17 studies29 (n=3,573) that assessed APRI, and found the area under the summary receiver operating characteristic curve to be 0.77, which is almost similar to the area under the receiver operating characteristic curve in our study of 0.723. Another meta-analysis of five studies found that a cut-off of 0.5 for APRI gave a specificity of 41%, while a cut-off of 1.5 of APRI gave a specificity of 84% for detection of significant fibrosis.28
After APRI, the next non-invasive parameter which became popular was FIB-4.15 In a meta-analysis29 of 10 studies assessing the FIB-4 for the prediction of significant fibrosis (n=1,996), the area under the summary receiver operating characteristic curve was 0.75, which is similar to the area under the receiver operating characteristic curve of 0.764 in our study.
APRI and FIB-4 have been compared in many previous studies and meta-analyses and FIB-4 was found to be slightly superior. In a study Lin et al.,30 FIB-4 and APRI were compared to evaluate their diagnostic values in identifying significant fibrosis and cirrhosis among 631 CHB patients. FIB-4 had a significantly higher area under the receiver operating characteristic curve than APRI to identify significant fibrosis and cirrhosis. Using FIB-4 outside the 0.87-3.40 range, significant fibrosis could be excluded in 69.2% of patients and cirrhosis could be diagnosed in 84.4%.30 Another meta-analysis of 39 studies found that the mean area under the summary receiver operating characteristic curve value of FIB-4 was higher than that of APRI (0.76 vs. 0.72) for predicting significant fibrosis.21 Similar results were shown by Houot et al.31 in their meta-analysis, where FIB-4 had better performance than APRI. A recent large study of almost 4000 patients (the SONIC-B study aimed at ruling-out cirrhosis) also found FIB-4 performing better than APRI.22 In contrast to these studies, a small Indian study found APRI to be superior to FIB-4 and Forn’s index. The study found NPV of APRI to be 95% for excluding significant liver fibrosis, while FIB-4 with a PPV of 61% showed fair correlation with significant fibrosis.32 Moreover, the World Health Organization recommend the use of APRI for estimating liver fibrosis in patients with CHB, where limited availability of resources was an issue.24
The next non-invasive parameter was GPR, which was developed in France and Western Africa to evaluate fibrosis in subjects with HBV, particularly in low-resource settings. The investigators had compared GPR with APRI and FIB-4 and found that the area under the receiver operating characteristic curve value of GPR was significantly superior to APRI and FIB-4 at identifying ≥F2 and ≥F3 in the African training and validation cohorts.23 Another comparative evaluation of GPR versus APRI and FIB-4 in predicting different levels of liver fibrosis of CHB also found that GPR had the best performance among the three. Using a cut-off of GPR >0.50 as standard, the sensitivities and specificities of GPR in predicting significant fibrosis in hepatitis B e antigen-positive patients were 59.6% and 81.2%, and those of hepatitis B e antigen-negative patients were 60.3% and 78.3% respectively. The authors suggested that this cut-off is almost similar to our cut-off of 0.444 for ruling-out significant fibrosis.33
The most recent of the non-invasive blood parameters assessed in our study was GAR, which was developed by Li et al.17 in 2017. The investigators had compared GAR to APRI and FIB-4 and had found GAR to have the highest area under the receiver operating characteristic curve for ≥F2, ≥F3, and ≥F4 fibrosis. The area under the receiver operating characteristic curve for GAR in our study was 0.734.
There are several limitations to our study. First, the performance of these non-invasive methods was assessed in a low fibrosis setting (13%), which is assumed to be reflective of the HBV population in India. Performance in higher fibrosis settings could be different from our results. Second, our results may not apply to patients in the immune-tolerant phase. Since this was a retrospective study conducted on patients who had undergone pre-treatment liver biopsy most patients in the immune-tolerant phase, who do not merit treatment, were excluded. Third, many confounding variables, such as coexisting obesity, metabolic syndrome and metabolic associated fatty liver disease, could have influenced the results. Fourth, GPR and GAR, both of which use GGT, can be affected by biliary tract disease and by some types of drugs, and this had not been evaluated in the reported studies. As such, our results of GPR and GAR need further evaluation in prospective studies.
In conclusion, we found that non-invasive blood parameters such as GPR, APRI, FIB-4 and GAR could be a useful parameters for screening of CHB patients who are at risk for developing liver fibrosis, especially in resource-poor settings and when TE is not available. Despite significant advances in developing non-invasive biomarkers that will help in evaluating hepatic fibrosis in patients with CHB, further large, prospective studies remain essential to validate accuracy, particularly for patients with mild hepatic fibrosis.34 In addition, a combination of these non-invasive biomarkers with or without TE may help to establish an algorithm to increase diagnostic accuracy of non-invasive assessment of liver fibrosis.
Abbreviations
- ALT
alanine aminotransferase
- APRI
aspartate aminotransferase-to-platelet ratio index
- AST
aspartate aminotransferase
- CHB
chronic hepatitis B
- FIB-4
Fibrosis-4
- GAR
gamma-glutamyl transpeptidase-to-albumin ratio
- GGT
gamma-glutamyl transpeptidase
- GPR
gamma-glutamyl transpeptidase-to-platelet ratio
- HBV
hepatitis B virus
- NPV
negative predictive value
- PPV
positive predictive value
- TE
transient elastography
References
- 1.World Health Organization Background-epidemiology and natural history. In: WHO. guidelines on hepatitis B and C testing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK442290/
- 2.Arora A, Singh SP, Kumar A, Saraswat VA, Aggarwal R, Bangar M, et al. INASL position statements on prevention, diagnosis and management of hepatitis B virus infection in India: The Andaman Statements. J Clin Exp Hepatol. 2018;8:58–80. doi: 10.1016/j.jceh.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMahon BJ. The natural history of chronic hepatitis B virus infection. McMahon BJ1. Hepatology. 2009;49:S45–S55. doi: 10.1002/hep.22898. [DOI] [PubMed] [Google Scholar]
- 4.Bárcena Marugán R, García Garzón S. DNA-guided hepatitis B treatment, viral load is essential, but not sufficient. World J Gastroenterol. 2009;15:423–430. doi: 10.3748/wjg.15.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar M, Sarin SK, Hissar S, Pande C, Sakhuja P, Sharma BC, et al. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT. Gastroenterology. 2008;134:1376–1384. doi: 10.1053/j.gastro.2008.02.075. [DOI] [PubMed] [Google Scholar]
- 6.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 7.Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15–20. [PubMed] [Google Scholar]
- 8.Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol. 2004;99:1160–1174. doi: 10.1111/j.1572-0241.2004.30110.x. [DOI] [PubMed] [Google Scholar]
- 9.Strassburg CP, Manns MP. Approaches to liver biopsy techniques--revisited. Semin Liver Dis. 2006;26:318–327. doi: 10.1055/s-2006-951599. [DOI] [PubMed] [Google Scholar]
- 10.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835–847. doi: 10.1016/j.jhep.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 13.Verma V, Sarin SK, Sharma P, Kumar A. Correlation of aspartate aminotransferase/platelet ratio index with hepatic venous pressure gradient in cirrhosis. United European Gastroenterol J. 2014;2:226–231. doi: 10.1177/2050640614527084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirnake V, Arora A, Sharma P, Goyal M, Chawlani R, Toshniwal J, et al. Non-invasive aspartate aminotransferase to platelet ratio index correlates well with invasive hepatic venous pressure gradient in cirrhosis. Indian J Gastroenterol. 2018;37:335–341. doi: 10.1007/s12664-018-0879-0. [DOI] [PubMed] [Google Scholar]
- 15.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 16.Vardar R, Vardar E, Demiri S, Sayhan SE, Bayol U, Yildiz C, et al. Is there any non-invasive marker replace the needle liver biopsy predictive for liver fibrosis, in patients with chronic hepatitis? Hepatogastroenterology. 2009;56:1459–1465. [PubMed] [Google Scholar]
- 17.Li Q, Lu C, Li W, Huang Y, Chen L. The gamma-glutamyl transpeptidase-to-albumin ratio predicts significant fibrosis and cirrhosis in chronic hepatitis B patients. J Viral Hepat. 2017;24:1143–1150. doi: 10.1111/jvh.12751. [DOI] [PubMed] [Google Scholar]
- 18.Teshale E, Lu M, Rupp LB, Holmberg SD, Moorman AC, Spradling P, et al. APRI and FIB-4 are good predictors of the stage of liver fibrosis in chronic hepatitis B: the Chronic Hepatitis Cohort Study (CHeCS) J Viral Hepat. 2014;21:917–920. doi: 10.1111/jvh.12279. [DOI] [PubMed] [Google Scholar]
- 19.Kim WR, Berg T, Asselah T, Flisiak R, Fung S, Gordon SC, et al. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J Hepatol. 2016;64:773–780. doi: 10.1016/j.jhep.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Cheng J, Hou J, Ding H, Chen G, Xie Q, Wang Y, et al. Validation of ten noninvasive diagnostic models for prediction of liver fibrosis in patients with chronic hepatitis B. PLoS One. 2015;10:e0144425. doi: 10.1371/journal.pone.0144425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology. 2015;61:292–302. doi: 10.1002/hep.27382. [DOI] [PubMed] [Google Scholar]
- 22.Sonneveld MJ, Brouwer WP, Chan HL, Piratvisuth T, Jia JD, Zeuzem S, et al. Optimisation of the use of APRI and FIB-4 to rule out cirrhosis in patients with chronic hepatitis B: results from the SONIC-B study. Lancet Gastroenterol Hepatol. 2019;4:538–544. doi: 10.1016/S2468-1253(19)30087-1. [DOI] [PubMed] [Google Scholar]
- 23.Lemoine M, Shimakawa Y, Nayagam S, Khalil M, Suso P, Lloyd J, et al. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut. 2016;65:1369–1376. doi: 10.1136/gutjnl-2015-309260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B. infection. 2015. Availbale from: https://www.ncbi.nlm.nih.gov/books/NBK305553/ [PubMed]
- 25.Degos F, Perez P, Roche B, Mahmoudi A, Asselineau J, Voitot H, et al. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study) J Hepatol. 2010;53:1013–1021. doi: 10.1016/j.jhep.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 26.Goyal R, Mallick SR, Mahanta M, Kedia S, Shalimar, Dhingra R, et al. Fibroscan can avoid liver biopsy in Indian patients with chronic hepatitis B. J Gastroenterol Hepatol. 2013;28:1738–1745. doi: 10.1111/jgh.12318. [DOI] [PubMed] [Google Scholar]
- 27.Castera L. Hepatitis B: are non-invasive markers of liver fibrosis reliable? Liver Int. 2014;34(Suppl 1):91–96. doi: 10.1111/liv.12393. [DOI] [PubMed] [Google Scholar]
- 28.Jin W, Lin Z, Xin Y, Jiang X, Dong Q, Xuan S. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis B-related fibrosis: a leading meta-analysis. BMC Gastroenterol. 2012;12:14. doi: 10.1186/1471-230X-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu XY, Kong H, Song RX, Zhai YH, Wu XF, Ai WS, et al. The effectiveness of noninvasive biomarkers to predict hepatitis B-related significant fibrosis and cirrhosis: a systematic review and meta-analysis of diagnostic test accuracy. PLoS One. 2014;9:e100182. doi: 10.1371/journal.pone.0100182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin CL, Liu CH, Wang CC, Liang CC, Su TH, Liu CJ, et al. Serum biomarkers predictive of significant fibrosis and cirrhosis in chronic hepatitis B. J Clin Gastroenterol. 2015;49:705–713. doi: 10.1097/MCG.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 31.Houot M, Ngo Y, Munteanu M, Marque S, Poynard T. Systematic review with meta-analysis: direct comparisons of biomarkers for the diagnosis of fibrosis in chronic hepatitis C and B. Aliment Pharmacol Ther. 2016;43:16–29. doi: 10.1111/apt.13446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shrivastava R, Sen S, Banerji D, Praharaj AK, Chopra GS, Gill SS. Assessment of non-invasive models for liver fibrosis in chronic hepatitis B virus related liver disease patients in resource limited settings. Indian J Pathol Microbiol. 2013;56:196–199. doi: 10.4103/0377-4929.120359. [DOI] [PubMed] [Google Scholar]
- 33.Liu DP, Lu W, Zhang ZQ, Wang YB, Ding RR, Zhou XL, et al. Comparative evaluation of GPR versus APRI and FIB-4 in predicting different levels of liver fibrosis of chronic hepatitis B. J Viral Hepat. 2018;25:581–589. doi: 10.1111/jvh.12842. [DOI] [PubMed] [Google Scholar]
- 34.Lin CL, Kao JH. Can noninvasive biomarkers replace liver biopsy for chronic hepatitis B? Hepatology. 2015;62:1924–1925. doi: 10.1002/hep.27865. [DOI] [PubMed] [Google Scholar]