Abstract
Ectopic ACTH-secretion causing Cushing's syndrome is unusual and its diagnosis is frequently challenging. The presence of high-molecular-weight precursors throughout pro-opiomelanocortin (POMC) translation by these tumors is often not reported. We present the case of a 49-year-old woman with a 3-month history of proximal muscular weakness, skin pigmentation, and weight loss. Upon initial evaluation, she had a full moon face, hirsutism, and a buffalo hump. Laboratory workup showed hyperglycemia, hypokalemia and metabolic alkalosis. ACTH, plasma cortisol, and urinary free cortisol levels were quite elevated. Serum cortisol levels were not suppressed on dexamethasone suppression testing. An octreo-SPECT scan showed enhanced nucleotide uptake in the liver and pancreas. Transendoscopic ultrasound-guided biopsy confirmed the diagnosis of a pancreatic ACTH-secreting neuroendocrine tumor (NET). Surgical excision of both pancreatic and liver lesions was carried out. Western blot analysis of the tumor and metastases revealed the presence of a high-molecular-weight precursor possibly POMC (at 30 kDa) but not ACTH (normally 4.5 kDa). ACTH-precursor secretion is more frequent in ectopic ACTH-secreting tumors compared with other causes of Cushing's syndrome. Hence, the measurement of such ACTH precursors warrants further evaluation, especially in the context of ACTH-dependent hypercortisolism.
Keywords: ACTH, ectopic, neuroendocrine tumor, cushing syndrome, case report
Introduction
Ectopic ACTH syndrome (EAS) is the cause of 5–10% of ACTH (adrenocorticotropic hormone) dependent Cushing's syndrome (1, 2). Ectopic ACTH syndrome can be a paraneoplastic manifestation of several kinds of neuroendocrine tumors, most commonly bronchial (well-differentiated carcinoid or poorly differentiated small cell), thymic, or pancreatic (3) but also occasionally pheochromocytoma, medullary thyroid carcinoma (MTC), and prostate carcinoma (3). Neuroendocrine tumors (NET) are heterogeneous in nature and can originate in the gastrointestinal tract, bronchi, thyroid and pancreas. The clinical presentation can range from neoplasms with indolent growth to rapidly advancing hormone-secreting carcinomas, and from occult to large symptomatic tumors (4). Early diagnosis and localization of the ectopic source of ACTH is crucial, in order to permit the complete excision of the tumor, avoiding adrenalectomy and reducing the risk of metastatic disease. However, in up to 50% of the cases, the source of ACTH secretion cannot be found despite imaging studies (5).
The diagnostic approach to ACTH dependent Cushing's syndrome (CS) relies on evaluating the clinical presentation, biochemical tests (including dynamic testing with dexamethasone), and inferior petrosal sinus sampling (IPSS). Inferior petrosal sinus sampling is the gold standard test for distinguishing between pituitary and ectopic ACTH-secreting tumors, this invasive procedure is not widely available and shows a specificity of only 67%, with false-positive and –negative results (6). The next step is the localization of EAS, this relies on conventional imaging techniques, including computed tomography (CT) and/or magnetic resonance imaging (MRI). Since ACTH-producing tumors express somatostatin receptor, somatostatin analogs (SSA) scintigraphy or gallium-68-somatostatin receptor PET/CT may also be helpful in localizing the tumor.
Additional tools, for the diagnosis of EAS, include the possibility of utilizing tumor specific differences in peptide processing. Some useful biomarkers, include pro-opiomelanocortin (POMC) and agouti-related protein (AgRP), especially in clinically challenging cases (7). AgRP is an hypothalamic neuropeptide that modulate energy balance, essential for energy homeostasis (8). Pro-opiomelanocortin is a complex precursor that includes various peptide hormones such as ACTH, melanocyte-stimulating hormone (MSH) alfa, and beta-endorphin (9). Pro-opiomelanocortin is a 241 amino acid protein with a molecular weight of 28–30 kDa. In EAS, there is often incomplete processing of POMC by the tumor, resulting in high serum levels of ACTH precursors (7).
In this report we describe the case of severe Cushing's syndrome caused by a metastatic pancreatic neuroendocrine tumor associated with the presence of high molecular weight ACTH-precursor molecules, possibly POMC, detected in the tumoral tissues.
Case Presentation
A 49-year-old Mexican, post-menopausal woman, of low socioeconomic status, was admitted to our institution with a 3-month history of proximal muscular weakness, skin hyperpigmentation, and bilateral ulcers on the lower limbs. She also mentioned unexplained weight loss (at least 6 kg) over the past 6 months. She had a past medical history of epilepsy, and a recent diagnosis of diabetes mellitus managed with metformin. She was not under further treatments or hormone replacement therapy. Physical examination revealed an overweight patient with a rounded “full moon” face, hirsutism (Figure 1C), mucocutaneous hyperpigmentation, a buffalo hump, upper extremity bruising, generalized weakness, and muscle wasting evident in the lower limbs. No skin striae where present. Thyroid examination was normal. Further clinical information is summarized in Table 1. The patient had significantly high ACTH [1,070 pg/mL (10–60)], plasma cortisol am [41.6 ug/dL (6–22.6)], and 24 h urinary cortisol levels [9,263 ug/24 h (<140), Table 2]. Other laboratory studies showed hyperglycemia [490 mg/dL (70–99)], low potassium levels [2.4 mmol/L (3.5–5.1)], and a metabolic alkalosis [bicarbonate 46.7 mmol/L (10–14), Table 1].
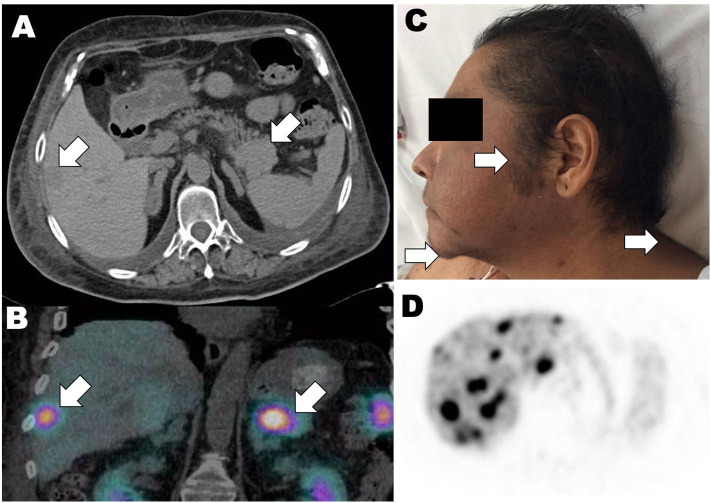
Figure 1.
(A) Computerized tomography showing neuroendocrine tumor at the pancreatic tail causing liver metastases (white arrows). (B) Octreotide-single-photon emission computerized tomography (SPECT) study showing hepatic and pancreatic focal lesions with enhanced uptake (white arrows). (C) Clinical picture of the patient remarking the skin hirsutism (white arrows), and hyperpigmentation. (D) 18-Fluorodeoxyglucose (18-FDG) positron emission tomography-computerized tomography (PET-CT) 18 months after diagnosis with persistence of tumor liver metastases (black spots).
Table 1.
Clinical and laboratory data at diagnosis.
| Parameter | Result | Reference ranges |
|---|---|---|
| Clinical data | ||
| Age | 49 years | – |
| Weight | 64 kg | – |
| BMI | 26.3 | – |
| Blood pressure | 100/60 | – |
| Heart rate | 128 | – |
| Respiratory rate | 28 | – |
| Temperature | 36.7°C | – |
| Laboratory data | ||
| Albumin | 3.2 | 3.5–5.7 mg/dL |
| pH | 7.51 | 7.31–7.41 |
| HCO3 | 46.7 | 24–28 mmol/L |
| PCO2 | 59 | 28–40 mmHg |
| HbA1C | 14 | <5.7% |
| DHEA-S | 41 | 35–430 ug/dL |
| DHEA | 3.5 | 0.2–9.8 ng/mL |
| Free testosterone | 62.3 | 0–2.03 pg/mL |
| Total testosterone | 3.6 | 0.1–0.75 pg/mL |
| Glucagon | 127 | 59–271 pg/mL |
| Chromogranin A | 5.7 | <3 nmol/L |
| Gastrin | 304 | 13–115 pg/mL |
| VIP | 11 | 0–30 pmol/L |
| CRH | 2.1 | <10 pg/mL |
ACTH, adrenocorticotropin; DHEA, dehydroepiandrosterone and DHEA-S, sulfate; VIP, vasoactive intestinal peptide; CRH, corticotropin releasing hormone.
Table 2.
Baseline cortisol and ACTH values and results of continuous 7 h IV infusion of 7 mg dexamethasone suppression test.
| On admission | Reference range | |
|---|---|---|
| Cortisol a.m. | 41.6 ug/dL | 6–22.6 ug/Dl |
| Cortisol p.m. | 60 ug/dL | 6–22.6 ug/dL |
| 24 h-urinary free cortisol | 9262.88 ug/24 h | <140 ug/24 h |
| ACTH | 1,070 pg/mL | 10–60 pg/mL |
| 7 mg dexamethasone infusion test (during 7 h) | ||
| Time before dexamethasone infusion started | ||
| Cortisol a.m.30 min (7:30 AM) | 41.66 ug/mL | Average: 41 ug/dl Normal <22 |
| Cortisol a.m.15 min (7:45 AM) | 41.18 ug/mL | |
| Cortisol a.m.0 min (8:00 AM) | 40.23 ug/dL | |
| Time before infusion of dexamethasone ended | ||
| Cortisol p.m.30 min (14:45 h) | 38.18 ug/dL | Average: 39 ug/dl Normal <10 |
| Cortisol p.m.15 min (15:00 h) | 40.53 ug/dL | |
| Cortisol p.m.0 min (15:15 h) | 38.94 ug/dL | |
| Next morning after dexamethasone infusion test | ||
| Cortisol a.m.0 min (8:00 AM) | 42.01 ug/dL | Average: 41 ug/dl Normal <10 |
| Cortisol a.m.15 min (8:15 AM) | 41.18 ug/dL | |
| Cortisol a.m.30 min (8:30 AM) | 40.68 ug/dL | |
| Laboratory hormone results after surgery | ||
| Plasma cortisol AM | 14.34 | 6–22.6 ug/dL |
| Plasma cortisol PM | 9.52 | 6–22.6 ug/dL |
| 24 h-urinary cortisol | 111.7 ug | <140 ug/day |
| ACTH | 24 | 10–60 pg/mL |
| DHEA | 0.51 | 0.2–9.8 ng/mL |
| Total testosterone | 0.17 | 0.1–0.75 pg/mL |
ACTH, adrenocorticotropin; DHEA, dehydroepiandrosterone.
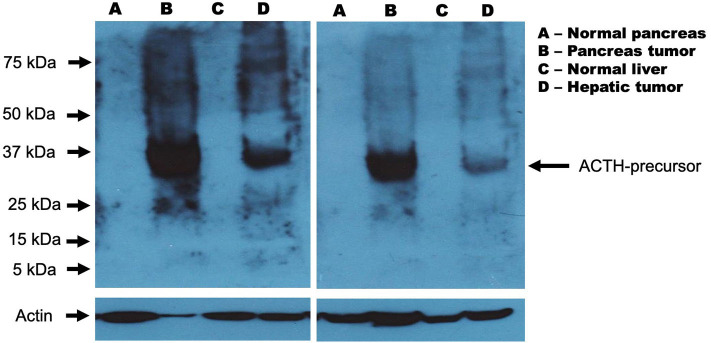
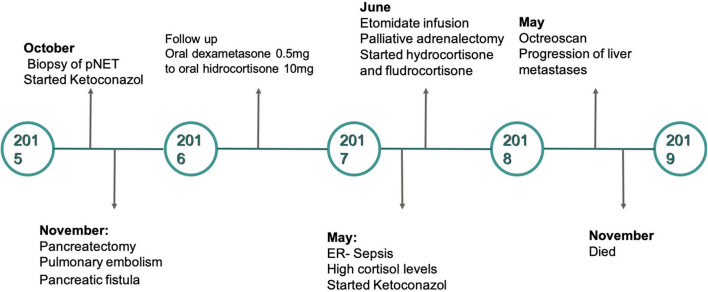
This prompted a workup to investigate the possibility of ACTH-dependent CS. A high-dose dexamethasone suppression test with a continuous 7 h IV infusion of 7 mg dexamethasone (1 mg/h, as described by Biemond and Bogaert) was carried out (15). Absence of cortisol suppression at morning of day 2 confirmed the presence of Cushing's syndrome, and, the lack of cortisol suppression at afternoon of day 1, clearly suggested an extra-pituitary source of hypercortisolism with a very low probability of a pituitary corticotroph adenoma (Table 2). Ectopic ACTH-secretion syndrome was therefore suspected. Diagnostic approach was completed with serum chromogranin A, total and free testosterone levels, all found elevated, while gastrin, VIP, CRH, dehydroepiandrosterone (DHEA), and DHEA-sulfate (DHEA-S) levels resulted on normal range (Table 1). Because rapid and aggressive onset of symptoms, the severity of the hypercortisolism (both clinically and biochemically), the high levels of ACTH, and the lack of suppression of cortisol at afternoon of day 1 during dexamethasone 7 mg infusion test (Table 2), the IPSS was not considered necessary in this case, as pituitary corticotroph adenoma was very unlikely. Instead, a computerized tomography study was performed, and result showed a 2.7 cm hypodense hepatic nodule, with a 4.3 cm distal pancreatic lesion, and bilateral diffuse adrenal hyperplasia (Figure 1A). The metabolic activity of these lesions was investigated using an octreotide-single-photon emission computerized tomography (SPECT) study. This showed increased metabolic activity in both tumors (Figure 1B). We considered the possibility of ectopic-ACTH secreting tumors at these sites. Therefore, the pancreatic lesion was biopsied using trans-endoscopic ultrasound (TEUS) and a well-differentiated pancreatic neuroendocrine tumor was confirmed. Surgery excision of both tumors was programmed. Preparation involved the use of ketoconazole, titrated up to 1,200 mg QD, in order to control cortisol levels before surgical excision. Distal pancreatectomy and liver metastasis resection were carried out without any acute complications. The pathology report established the diagnosis of a grade 2 well-differentiated neuroendocrine pancreatic neoplasm positive for chromogranin A, synaptophysin, and Ki-67 (15%) (16). In order to confirmed ACTH synthesis from both pancreas and liver tumors, we performed western blot (WB) analysis for ACTH, on both human histological samples obtained from the patient. Results showed a 30 kDa protein in both pancreatic and liver tumors, but negative on normal pancreas and liver as control (Figure 2). Also, WB did not show any protein at ACTH molecular weight of 4.5 kDa. Taking all together, WB results suggested the presence of a high molecular weight ACTH-precursor such as POMC (Figure 2).
Figure 2.
Western blot analysis from samples of pancreatic tumor and liver metastasis. (A) Normal pancreas; (B) Pancreatic NET; (C) Normal liver; (D) Liver metastasis. Pancreatic and liver tissues were homogenized in the presence of RIPA buffer (PBS with detergents and protease inhibitors cocktail). Total protein concentration was determined using the commercial Bradford reagent assay (Bio-Rad, Hercules, CA). Whole protein (200 μg) was used for the detection of the ACTH protein. Samples were first boiled in sample buffer (125 mM Tris-HCl, pH 6.8, 1% v/w SDS, 10% v/v glycerol, 0.1% bromophenol blue, 2% v/v 2 alfa-mercaptoethanol) for 5 min and separated by 12% SDS-PAGE. Then, the gels were transferred to PVDF membranes (Merck Millipore Corporation, Darmstadt Germany) using a Trans-Blot Cell system (Bio-Rad, Hercules, CA) in transfer buffer (25 mM Tris, 190 mM glycine, and 10% methanol) at 40 V overnight. The following day, the membranes were blocked with non-fat dried milk (5%) dissolved in TBS buffer (150 mM NaCl, 20 mM Tris, and 0.1% Tween) and probed overnight at 4 °C with rabbit monoclonal anti-ACTH antibody (Abcam, Cambridge UK) diluted 1:2,000 in TBS buffer with BSA (150 mM NaCl, 20 mM Tris, 0.1% Tween, and 1% BSA at pH 7.5). After washing, the membranes were incubated for 1 h with anti-rabbit immunoglobulin-HRP (Thermo Scientific, Rockford, IL USA). The signals were detected by enhanced chemiluminescence using the SuperSignalTM system (Thermo Scientific, Rockford, IL USA) on X-ray film (Kodak, USA). As the control loaded, actin was simultaneously detected, using mouse anti-human β-actin antibody (Santa Cruz Biotechnology, Santa Cruz CA, USA). The signal was developed using anti-mouse immunoglobulin-HRP (Thermo Scientific, Rockford, IL) and chemiluminescence system.
Ketoconazole was gradually withdrawn after surgery, and corticosteroid replacement therapy with hydrocortisone was provided for 3 days after the surgical intervention. Morning and afternoon cortisol levels, as well as 24-hour urinary free cortisol were measured and were reported in the normal range (Table 2). Unfortunately, postoperative hospitalization was complicated by a pancreatic fistula, pulmonary embolism, and hospital acquired pneumonia. Conservative management for the pancreatic fistula was undertaken. The pulmonary embolism and pneumonia were appropriately treated and resolved. The patient was discharged with an outpatient visit scheduled within a month.
Following 18 months of ambulatory follow-up, the patient was admitted to the emergency room with diffuse abdominal pain, vomiting, and muscle weakness. High ACTH (1,000 pg/ml), morning cortisol levels (62 ug/dl), UFC (3,509 ug/24 h), together with hypokalaemia (3.08 mmol/L) were recorded. An urgent bilateral adrenalectomy was programmed to definitively control the hypercortisolism. In preparation for the surgery, an etomidate infusion was started (initial dose 0.04 mg/kg/h) and titrated to control cortisol levels (using daily 24-h urinary cortisol and plasma cortisol levels). The dose of the drug was then gradually decreased. The day before surgery, the infusion was stopped; and 8 h before surgery, hydrocortisone replacement therapy was started using 100 mg IV every 8 h. The adrenalectomy was carried out with no complications. Following the surgical procedure, prednisone with fludrocortisone replacement were started. The clinical picture of patient improved; however, 18-fluorodeoxyglucose (18-FDG) positron emission tomography-computerized tomography (PET-CT) confirmed the presence of inoperable liver metastases (Figure 1D). No further surgical intervention could be offered, and the patient eventually died. Her survival following the initial diagnosis was no >36 months (Figure 3). Written informed consent was obtained from the patient for this report. Nevertheless, her clinical perspective was not obtained.
Figure 3.
Timeline of the case presented.
Discussion
Ectopic ACTH syndrome (EAS) represents one of the major diagnostic challenges in clinical endocrinology (17–19), regardless of the availability of broad diagnostic tests. In nearly half of the cases, the source of ectopic ACTH-secretion is situated in the lung, bronchial carcinoid tumors being the most common cause (20, 21). ACTH-secreting pancreatic neuroendocrine tumors are rare and more than 95% metastasize (22). As in the case of our patient, such neuroendocrine tumors usually present with liver metastasis since neoplastic cells from the pancreas enter the enterohepatic circulation (23) and plasma chromogranin A (CgA) is usually elevated (24).
An effort has been made to describe the clinical and pathologic features of ACTH-secreting pancreatic neuroendocrine tumors despite its rarity (25). Maragliano reviewed 134 cases (124 previously reported and 10 from their series), reporting a female preponderance (66%), occurring at a mean age of 42.1 years. This age average is almost 20 years less than the mean age compared to the overall pancreatic neuroendocrine tumors (26). More than a third of the cases, clinical presentation is accompanied with Zollinger-Ellison syndrome whereas only 5% presents with insulinoma, and 1% with carcinoid syndrome (25). Although serum CgA has been widely used as a diagnostic biomarker in pancreatic neuroendocrine tumors, it has a limited role, especially with localized diseases (27). However, serum alfa-feto protein elevation should prompt consideration of the presence of pancreatic acinar cell carcinoma and/or pancreatoblastomas since it is associated with worst prognosis (25). Radiolabeled somatostatin analogs diagnostic imaging such as PET/CT with gallium-68 DOTATATE is used for most patients in which the uptake of radiolabeled somatostatin analogs can anticipate the clinical response to therapy (28).
It has been suggested that the signs and symptoms of EAS are influenced by the tissue and type of tumor secreting ACTH (10). Pancreatic neuroendocrine neoplasms associated with EAS generally show a short time between the onset of clinical symptoms of hypercortisolism and diagnosis (6, 17). Our patient was diagnosed promptly, and rapidly underwent surgical treatment to control hypercortisolism. Aggressive EAS, can be associated with high POMC/ACTH ratio since the tumor tissue may secrete precursors that are not detected in conventional ACTH assays (11, 12). However, we were not able to evaluate POMC serum levels. First, we do not perform this evaluation as routine, and therefore, we did not have the assay ready throughout the diagnostic approach, and secondly, aggressiveness of the Cushing's syndrome required promptly medical and surgical therapy, and serum samples were not preserved before treatment was started. Instead, western blot was carried out as an effort to evaluate the presence of ACTH precursor molecules on this case. Figure 2 shows strong positiveness of a 30-kDa protein isolated from the pancreatic and hepatic tumor tissues, but not at their normal controls. ACTH is a 39 amino acid peptide with a 4.5 kDa molecular mass, synthesized from pre-pro-opiomelanocortin (pre-POMC). During translation, the signal peptide is removed and produces a 241-amino acid polypeptide POMC. After multiple post-translational modifications, POMC is proteolytically cleaved from amino acid 135–174 to synthesize ACTH (13). Using an immunoradiometric assay for the quantitative determination of whole corticotropic hormone (ACTH) in human plasma (ELSA-ACTH Cisbio Bioassays, Model 22), two monoclonal antibodies are prepared against sterically remote antigenic sites on the ACTH molecule. Detected serum ACTH levels in our patient, however, may in fact reflect quantification of same antigenic sites but from amino acid 135–174 at the ACTH-precursor POMC. Otherwise, it would be very difficult to explain the absence of ACTH on western blot from both tumors (pancreas and liver) in the context of a patient with an aggressive ACTH-ectopic secretion Cushing syndrome. In fact, cross-reactivity in ACTH assay with ACTH precursors have been reported up to 10% (14, 29, 30). We, therefore, can assess the possibility that POMC together with other intermediary proteins throughout POMC translation such as melanocyte-stimulating hormone alfa (MSH-alfa), contributed to the aggressive clinical presentation in our patient. Furthermore, the clinical utility of serum POMC levels have been demonstrated in differentiated Cushing disease and occult EAS, particularly when there is no detectable tumor or there is a pituitary microadenoma on MRI and IPSS is required (7, 31–34). Moreover, increased serum levels of AgRP can also be considered as a neuroendocrine tumor marker for EAS (7). Ectopic ACTH secretion associated with underlying malignancy is treated with surgery. Prognosis depends on the histological findings and the presence of metastasis. Also, regardless of primary lesion resection, and control of secreted hormone levels and clinical manifestations, recurrence rate is high, particularly, with liver metastases as in the case of our patient (35).
Non-surgical liver-directed therapies include ablation and hepatic arterial embolization. Ablation are mainly employed for liver metastasis or as an adjunct for surgical treatment or resection whereas hepatic arterial embolization, as a palliative care for symptomatic, non-resectable hepatic disease or an alternative for medical therapy (36). Cytotoxic chemotherapy, including capecitabine and temozolomide, are recommended in symptomatic patients from tumor mass or in the presence of a fast-growing metastasis (36). Therapeutic targets for neuroendocrine tumors include phosphatidylinositol-3-kinase (PI3K)/Akt and rapamycin (mTOR), both of which are involved in cell-cycle and growth control pathways in cancer (37, 38). Also, everolimus, an oral inhibitor of the mTOR pathway, and sunitinib, an oral multi-targeted tyrosine kinases inhibitor have been shown to improve progression-free survival in metastatic entero-pancreatic neuroendocrine tumors (39, 40). Lanreotide and octreotide, which are somatostatin analogs that act by binding to somatostatin type 2 receptors, are used to control symptoms. In addition, lanreotide prolongs progression-free survival in pancreatic NETs (41). Recently, peptide receptor-targeted radiotherapy (PRRT) in the treatment of metastatic NETs with a homogenous expression of SSTRs has been tested, mainly using 90Yttrium- DOTA-TOC or 177Lutetium- DOTA-TATE (42, 43). Response rates of 15–40% have been reported, although prospective randomized clinical trials comparing these agents with conventional treatment are lacking. Although the majority of NETs growth slowly with a relatively indolent course, up to 40% may show metastases at the time of diagnosis. Even in the presence of liver metastases, such as the case presented here, the majority of such patients may survive for many years with the use of these emerging therapies.
Although prognosis are variable, there are several factors that impact survival, including age, type of NET, histologic grade, hypercortisolism and metastases (3, 17, 44). In a series of NETs and EAS, pancreatic NETs had a lower curative surgical rate and a lower 5-year overall survival when compared to bronchial carcinoid EAS (45). The median survival is 50 months in patients with grade one or two pancreatic NETs and distant metastases (44).
To conclude, although we could not measure serum level of POMC, we were able to demonstrate its presence in two different tumor tissues (liver and pancreas) from a patient with an aggressive Cushing's syndrome. This case emphasizes the relationship between ectopic ACTH-secreting Cushing's syndrome and importance of ACTH precursors quantification such as POMC. The measurement of such precursors warrants further evaluation, especially in the workup of such cases.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Written informed consent has been obtained from the patient for publication of the submitted article and images.
Author Contributions
RM, CL-C, PR-S, GV-R, OJ-L, JL-D, MG-G, AL-S, and DC-R: diagnostic approach of the case, sample collection, writing the paper, editing images, and literature review. MG-P: diagnostic approach of the case, writing the paper, editing images, and literature review. JH-A: writing the paper, editing images, and literature review. JV-G: sample collection, preparation, and test. FG-P: diagnostic approach of the case, editing images, and literature review. All authors: read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing's syndrome. Lancet. (2006) 367:1605–17. 10.1016/S0140-6736(06)68699-6 [DOI] [PubMed] [Google Scholar]
- 2.Sathyakumar S, Paul TV, Asha HS, Gnanamuthu BR, Paul MJ, Abraham DT, et al. Ectopic cushing syndrome: a 10-year experience from a tertiary care center in Southern India. Endocr Pract. (2017) 23:907–14. 10.4158/EP161677.OR [DOI] [PubMed] [Google Scholar]
- 3.Kamp K, Alwani RA, Korpershoek E, Franssen GJ, de Herder WW, Feelders RA. Prevalence and clinical features of the ectopic ACTH syndrome in patients with gastroenteropancreatic and thoracic neuroendocrine tumors. Eur J Endocr. (2016) 174:271–80. 10.1530/EJE-15-0968 [DOI] [PubMed] [Google Scholar]
- 4.Ducry J, Gomez F, Prior JO, Boubaker A, Matter M, Monti M, et al. Mid-gut ACTH-secreting neuroendocrine tumor unmasked with (18)F-dihydroxyphenylalanine-positron emission tomography. Endocrinol Diabetes Metab Case Rep. (2015) 2015:140104. 10.1530/EDM-14-0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Herder WW, Lamberts SW. Tumor localization–the ectopic ACTH syndrome. J Clin Endocrinol Metab. (1999) 84:1184–5. 10.1210/jc.84.4.1184 [DOI] [PubMed] [Google Scholar]
- 6.Isidori AM, Sbardella E, Zatelli MC, Boschetti M, Vitale G, Colao A, et al. Conventional and nuclear medicine imaging in ectopic cushing's syndrome: a systematic review. J Clin Endocrinol Metab. (2015) 100:3231–44. 10.1210/JC.2015-1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Page-Wilson G, Freda PU, Jacobs TP, Khandji AG, Bruce JN, Foo ST, et al. Clinical utility of plasma POMC and AgRP measurements in the differential diagnosis of ACTH-dependent Cushing's syndrome. J Clin Endocrinol Metab. (2014) 99:E1838–45. 10.1210/jc.2014-1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilnytska O, Argyropoulos G. The role of the Agouti-related protein in energy balance regulation. Cellul Mol Life Sci. (2008) 65:2721–31. 10.1007/s00018-008-8104-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi A, Amemiya Y, Nozaki M, Sower SA, Joss J, Gorbman A, et al. Isolation and characterization of melanotropins from lamprey pituitary glands. Int J Peptide Protein Res. (1995) 46:197–204. 10.1111/j.1399-3011.1995.tb00589.x [DOI] [PubMed] [Google Scholar]
- 10.Alexandraki KI, Grossman AB. The ectopic ACTH syndrome. Rev Endocr Metab Disord. (2010) 11:117–26. 10.1007/s11154-010-9139-z [DOI] [PubMed] [Google Scholar]
- 11.Tabarin A, Corcuff JB, Rashedi M, Navarranne A, Ducassou D, Roger P. Comparative value of plasma ACTH and beta-endorphin measurement with three different commercial kits for the etiological diagnosis of ACTH-dependent Cushing's syndrome. Acta Endocr. (1992) 126:308–14. 10.1530/acta.0.1260308 [DOI] [PubMed] [Google Scholar]
- 12.Raffin-Sanson ML, Massias JF, Dumont C, Raux-Demay MC, Proeschel MF, Luton JP, et al. High plasma proopiomelanocortin in aggressive adrenocorticotropin-secreting tumors. J Clin Endocrinol Metab. (1996) 81:4272–7. 10.1210/jcem.81.12.8954027 [DOI] [PubMed] [Google Scholar]
- 13.Gibson S, Pollock A, Littley M, Shalet S, White A. Advantages of IRMA over RIA in the measurement of ACTH. Ann Clin Biochem. (1989) 26(Pt 6):500–7. 10.1177/000456328902600608 [DOI] [PubMed] [Google Scholar]
- 14.Monaghan PJ, Kyriacou A, Sturgeon C, Davies A, Trainer PJ, White A, et al. Proopiomelanocortin interference in the measurement of adrenocorticotrophic hormone: a United Kingdom National external quality assessment service study. Clin Endocrinol. (2016) 85:569–74. 10.1111/cen.13118 [DOI] [PubMed] [Google Scholar]
- 15.van den Bogaert DP, de Herder WW, de Jong FH, Biemond P, van der Lely AJ, Lamberts SW. The continuous 7-hour intravenous dexamethasone suppression test in the differential diagnosis of ACTH-dependent cushing's syndrome. Clin Endocrinol. (1999) 51:193–8. 10.1046/j.1365-2265.1999.00759.x [DOI] [PubMed] [Google Scholar]
- 16.Inzani F, Petrone G, Rindi G. The new world health organization classification for pancreatic neuroendocrine Neoplasia. Endocrinol Metab Clin North Am. (2018) 47:463–70. 10.1016/j.ecl.2018.04.008 [DOI] [PubMed] [Google Scholar]
- 17.Isidori AM, Kaltsas GA, Pozza C, Frajese V, Newell-Price J, Reznek RH, et al. The ectopic adrenocorticotropin syndrome: clinical features, diagnosis, management, and long-term follow-up. J Clin Endocrinol Metab. (2006) 91:371–7. 10.1210/jc.2005-1542 [DOI] [PubMed] [Google Scholar]
- 18.de Matos LL, Trufelli DC, das Neves-Pereira JC, Danel C, Riquet M. Cushing's syndrome secondary to bronchopulmonary carcinoid tumor: report of two cases and literature review. Lung Cancer. (2006) 53:381–6. 10.1016/j.lungcan.2006.05.019 [DOI] [PubMed] [Google Scholar]
- 19.Menezes Nunes J, Pinho E, Camões I, Maciel J, Cabral Bastos P, Souto de Moura C, et al. A challenging case of an ectopic cushing syndrome. Case Rep Med. (2014) 2014:413136. 10.1155/2014/413136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scanagatta P, Montresor E, Pergher S, Mainente M, Bonadiman C, Benato C, et al. Cushing's syndrome induced by bronchopulmonary carcinoid tumours: a review of 98 cases and our experience of two cases. Chir Ital. (2004) 56:63–70. [PubMed] [Google Scholar]
- 21.Ejaz S, Vassilopoulou-Sellin R, Busaidy NL, Hu MI, Waguespack SG, Jimenez C, et al. Cushing syndrome secondary to ectopic adrenocorticotropic hormone secretion: the University of Texas MD Anderson cancer center experience. Cancer. (2011) 117:4381–9. 10.1002/cncr.26029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen RT, Cadiot G, Brandi ML, de Herder WW, Kaltsas G, Komminoth P, et al. ENETS Consensus guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumor syndromes. Neuroendocrinology. (2012) 95:98–119. 10.1159/000335591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao WQ, Wu X, Li GD, Wu WL, Wang WY. ACTH-secreting pancreatic neuroendocrine carcinoma with ovarian and pelvic metastases causing Cushing's syndrome: a case report. Int J Clin Exp Pathol. (2015) 8:15396–401. [PMC free article] [PubMed] [Google Scholar]
- 24.Jun E, Kim SC, Song KB, Hwang DW, Lee JH, Shin SH, et al. Diagnostic value of chromogranin A in pancreatic neuroendocrine tumors depends on tumor size: a prospective observational study from a single institute. Surgery. (2017) 162:120–30. 10.1016/j.surg.2017.01.019 [DOI] [PubMed] [Google Scholar]
- 25.Maragliano R, Vanoli A, Albarello L, Milione M, Basturk O, Klimstra DS, et al. ACTH-secreting pancreatic neoplasms associated with Cushing syndrome: clinicopathologic study of 11 cases and review of the literature. Am J Surg Pathol. (2015) 39:374–82. 10.1097/PAS.0000000000000340 [DOI] [PubMed] [Google Scholar]
- 26.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. (2008) 26:3063–72. 10.1200/JCO.2007.15.4377 [DOI] [PubMed] [Google Scholar]
- 27.Pulvirenti A, Rao D, McIntyre CA, Gonen M, Tang LH, Klimstra DS, et al. Limited role of chromogranin A as clinical biomarker for pancreatic neuroendocrine tumors. HPB. (2019) 21:612–8. 10.1016/j.hpb.2018.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadowski SM, Neychev V, Millo C, Shih J, Nilubol N, Herscovitch P, et al. Prospective study of 68Ga-DOTATATE positron emission tomography/computed tomography for detecting gastro-entero-pancreatic neuroendocrine tumors and unknown primary sites. J Clin Oncol. (2016) 34:588–96. 10.1200/JCO.2015.64.0987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talbot JA, Kane JW, White A. Analytical and clinical aspects of adrenocorticotrophin determination. Ann Clin Biochem. (2003) 40(Pt 5):453–71. 10.1258/000456303322326371 [DOI] [PubMed] [Google Scholar]
- 30.Crosby SR, Stewart MF, Ratcliffe JG, White A. Direct measurement of the precursors of adrenocorticotropin in human plasma by two-site immunoradiometric assay. J Clin Endocrinol Metab. (1988) 67:1272–7. 10.1210/jcem-67-6-1272 [DOI] [PubMed] [Google Scholar]
- 31.White A, Gibson S. ACTH precursors: biological significance and clinical relevance. Clin Endocrinol. (1998) 48:251–5. 10.1046/j.1365-2265.1998.00451.x [DOI] [PubMed] [Google Scholar]
- 32.Stewart PM, Gibson S, Crosby SR, Penn R, Holder R, Ferry D, et al. ACTH precursors characterize the ectopic ACTH syndrome. Clin Endocrinol. (1994) 40:199–204. 10.1111/j.1365-2265.1994.tb02468.x [DOI] [PubMed] [Google Scholar]
- 33.Oliver RL, Davis JR, White A. Characterisation of ACTH related peptides in ectopic Cushing's syndrome. Pituitary. (2003) 6:119–26. 10.1023/B:PITU.0000011172.26649.df [DOI] [PubMed] [Google Scholar]
- 34.Ratter SJ, Gillies G, Hope J, Hale AC, Grossman A, Gaillard R, et al. Pro-opiocortin related peptides in human pituitary and ectopic ACTH secreting tumours. Clin Endocrinol. (1983) 18:211–8. 10.1111/j.1365-2265.1983.tb03205.x [DOI] [PubMed] [Google Scholar]
- 35.Kim H, Song KB, Hwang DW, Lee JH, Alshammary S, Kim SC. Time-trend and recurrence analysis of pancreatic neuroendocrine tumors. Endocr Connect. (2019) 8:1052–60. 10.1530/EC-19-0282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uri I, Grozinsky-Glasberg S. Current treatment strategies for patients with advanced gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Clin Diabetes Endocrinol. (2018) 4:16. 10.1186/s40842-018-0066-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolin EM. PI3K/Akt/mTOR pathway inhibitors in the therapy of pancreatic neuroendocrine tumors. Cancer Lett. (2013) 335:1–8. 10.1016/j.canlet.2013.02.016 [DOI] [PubMed] [Google Scholar]
- 38.Chan J, Kulke M. Targeting the mTOR signaling pathway in neuroendocrine tumors. Curr Treat Opt Oncol. (2014) 15:365–79. 10.1007/s11864-014-0294-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. (2011) 364:501–13. 10.1056/NEJMoa1003825 [DOI] [PubMed] [Google Scholar]
- 40.Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. (2011) 364:514–23. 10.1056/NEJMoa1009290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caplin ME, Pavel M, Cwikła JB, Phan AT, Raderer M, Sedláčková E, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. (2014) 371:224–33. 10.1056/NEJMoa1316158 [DOI] [PubMed] [Google Scholar]
- 42.Kwekkeboom DJ, Krenning EP. Peptide receptor radionuclide therapy in the treatment of neuroendocrine tumors. Hematol Oncol Clin North Am. (2016) 30:179–91. 10.1016/j.hoc.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 43.Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, et al. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology. (2016) 103:153–71. 10.1159/000443171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. (2017) 3:1335–42. 10.1001/jamaoncol.2017.0589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davi' MV, Cosaro E, Piacentini S, Reimondo G, Albiger N, Arnaldi G, et al. Prognostic factors in ectopic Cushing's syndrome due to neuroendocrine tumors: a multicenter study. Eur J Endocrinol. (2017) 176:453–61. 10.1530/EJE-16-0809 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.