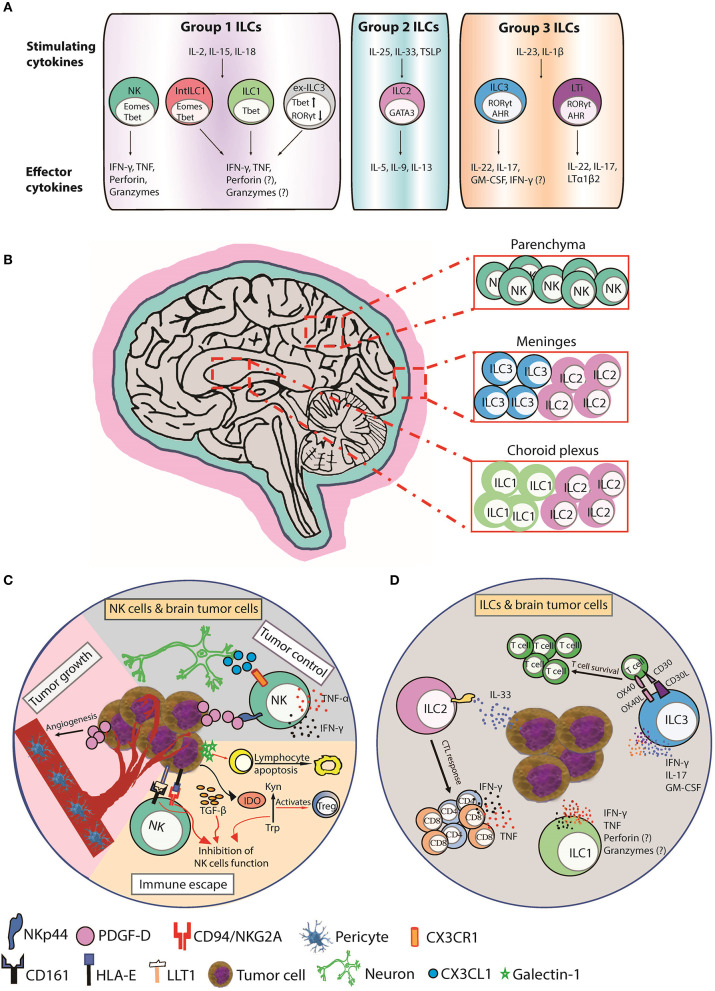
Figure 1.
Function, distribution, and anti-tumor responses of CNS ILC subsets. (A) Group 1 CNS ILCs have thus far been shown to include NK cells, intermediate ILC1s (intILC1s), ILC1s, and “ex-ILC3s.” NK cells express T-bet and Eomes and secrete IFN-γ and TNF in response to IL-2, IL-15, and IL-18, and lyse malignant cells via perforin, and granzymes. ILC1s express T-bet and produce IFN-γ and TNF in response to IL-2, IL-15, and IL-18 to promote type I immunity. Intermediate ILC1 (IntILC1) represent an intermediate phenotype between NK and ILC1 and express T-bet and Eomes. Ex-ILC3s are former ILC3 that have upregulated T-bet and downregulated RORγt to differentiate into ILC1-like cells. ILC2s express GATA3 and secrete IL-5, IL-9, and IL-13 in response to IL-25, IL-33, and TSLP to promote type II immunity. Group 3 ILCs include ILC3s and LTi cells. ILC3s express RORγt and AHR and produce IL-17, IL-22, and GM-CSF in respond to IL-23 and IL-1β stimulation to counteract extracellular bacterial and fungal infections. LTi cells also express RORγt and AHR and produce IL-17, IL-22, and lymphotoxin (LTα1β2). LTi cells trigger lymphoid tissue organogenesis during development. (B) NK cells are mainly present in the brain parenchyma. ILC1s are enriched in choroid plexus, whereas ILC2s accumulate in the choroid plexus and meninges and ILC3s accumulate in the meninges. (C) CX3CR1+ NK cells can infiltrate the brain in response to CX3CL1 chemokine produced by neurons. (Tumor control) PDGF-D expressed by tumor cells binds to the activating NKp44 receptor expressed on activated NK and induces the secretion of IFN-γ and TNF that inhibits tumor cell proliferation. (Tumor growth) PDGF-D enhances tumor growth by promoting pericyte recruitment and tumor angiogenesis. (Immune escape) Tumor cells upregulate IDO, which inactivates NK cells and activates immunosuppressive regulatory T cells (Tregs) by depletion of Trp and accumulation of Kyn. Tumor cells also secrete galectin-1 that induces lymphocyte apoptosis. Tumor cells supress NK cells function by inducing HLA-E and LLT1 ligands, which are ligands for NK cell inhibitory receptors CD94/NKG2A and CD161, respectively. TGF-β also inhibits NK cells function. (D) A hypothetical scheme showing the possible role of ILCs in brain cancer. Human ILC1s and ILC3s can express NKp44 and secrete IFN-γ and/or TNF in response to PDGF-DD to promote anti-tumor immunity (2). ILC2s enhance CTL responses to control the spread of tumors in response to IL-33 produced by tumor cells. ILC3s can also produce proinflammatory cytokines IFN-γ, IL-17, and GM-CSF and express the costimulatory molecules CD30L and OX40L that can promote T cell survival and function. AHR, aryl hydrocarbon receptor; CTL, cytotoxic T lymphocyte; EOMES, eomesodermin; GATA3, GATA-binding protein 3; GM-CSF, granulocyte-macrophage colony-stimulating factor; IDO, indoleamine 2,3-dioxygenase; Kyn, kynurenine; LLT1, Lectin-like transcript-1; LTi, Lymphoid tissue-inducer; PDGF-D, Platelet Derived Growth Factor D; RORγt, retinoic acid-related orphan receptor gamma t; T-bet, T-box expressed in T cells; Trp, tryptophan; TSLP, thymic stromal lymphopoietin.