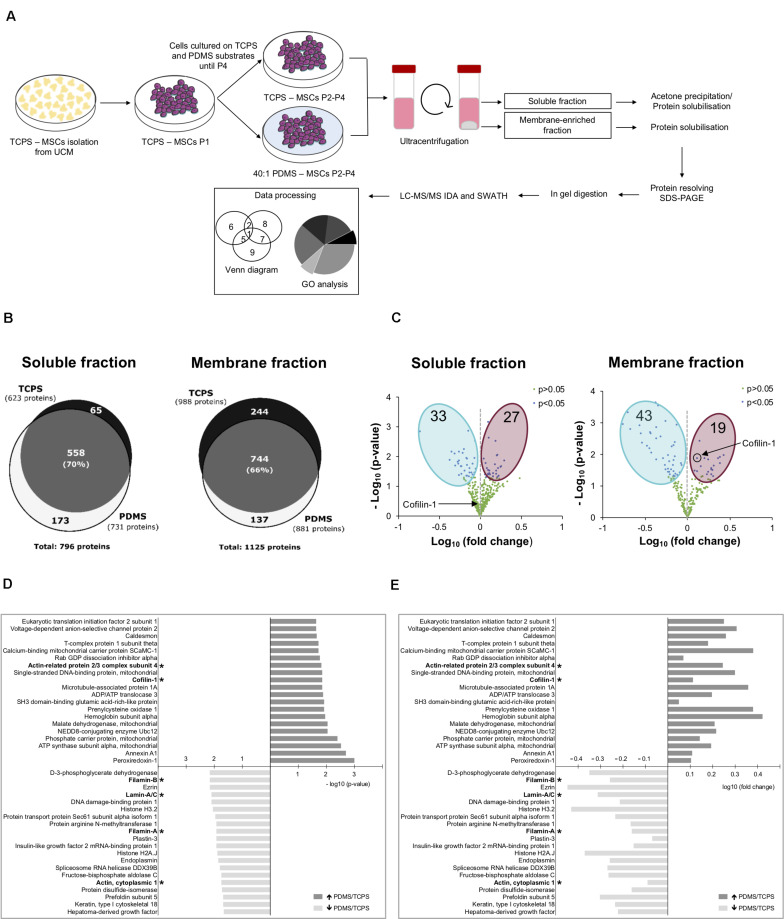
FIGURE 2.
Substrate stiffness influences the proteome of hUCM-MSCs. (A) Experimental workflow. hUCM-MSCs were isolated from umbilical cord explants obtained from three distinct donors on TCPS plates and passaged (into P1) when the colonies were well developed. Cells were then maintained in parallel in culture from P2 until P4 on stiff TCPS and soft 40:1 PDMS substrates. Cells were lysed and then the membrane- and soluble-enriched fractions were obtained by ultra-centrifugation. The proteins from the two fractions were precipitated and/or solubilised, resolved by SDS-PAGE and analysed by mass spectrometry. (B) Venn diagrams illustrating the total number of proteins and the number of exclusive and common proteins identified in cells cultured on TCPS or 40:1 PDMS present in the soluble (left) or membrane (right) enriched fractions. (C) Volcano plots representing proteins with statistically significant differences (p < 0.05, blue dots) or non-significant differences (p > 0.05, green dots) between proteins found in the proteome of soluble (left) and membrane (right) fractions obtained from cells cultured on soft PDMS substrates relative to stiff TCPS. The blue and magenta areas surround the proteins with statistically significant lower or higher expression in cells cultured on soft PDMS versus stiff TCPS, respectively. Black circle and arrows pinpoint Cofilin-1 found in soluble (left) and membrane (right) fractions of cells cultured on soft PDMS versus stiff TCPS. Statistical analysis was performed using One-Sample t-test (theoretical mean of 1.0). (D) Bar chart representing the top 20 most significant (p < 0.05) and (E) differentially abundant proteins (fold change) present in soluble and membrane fractions obtained from cells cultured on 40:1 PDMS versus TCPS substrates. (*) marks proteins involved in mechanotransduction or actin cytoskeleton regulation. Bars represent –log10 of the p-value (D) or log10 of the fold change (E). All data were collected from three independent experiments using cells obtained from three distinct donors.