Fig. 1. Water adsorption and condensation on gibbsite nanoparticles.
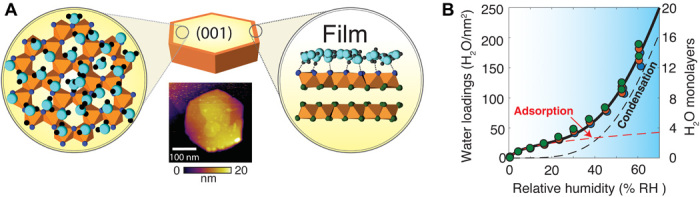
(A) Schematic representation of a water film on the basal (001) face of a single gibbsite (γ-Al(OH)3) nanoparticle. Molecular structures are taken from a snapshot of a selected portion of a pristine simulation cell (see Fig. 5 at 12 H2O/nm2) by molecular dynamics (MD). Water molecules (turquoise O, black H) form networks of hydrogen bonds (dashed line) with surface hydroxo groups of the (001) surface. AFM imaging revealed the morphology, crystal habit, topographical variations, and defects of the gibbsite nanoparticles under study (see fig. S1 for additional images). (B) Water vapor adsorption isotherms (25°C) on gibbsite nanoparticles measured by quartz crystal microgravimetry. A modified water binding model (Eq. 2; see Materials and Methods) predicts adsorption data (here collected in triplicates) in terms of an adsorption regime at low % RH and of a condensation regime at high % RH.
