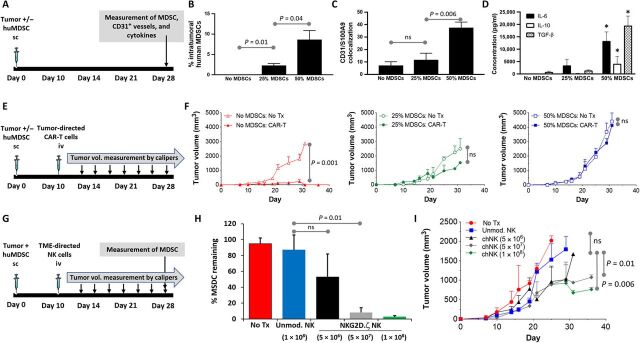
Fig. 1. Elimination of intratumoral MDSCs does not correlate with tumor regression.
(A) Treatment schema for experiments assessing MDSC dose-dependent tumor burden, vessel density, and suppressive environment. (B) Tumors were harvested en bloc, and percentage of MDSCs within tumors inoculated alone (No MDSCs) or with a 25 or 50% MDSC inoculation dose was determined by flow cytometry. (C) Tumors were harvested, sectioned, and analyzed (n = 5 samples per section) for presence of microvasculature by human CD31 immunostaining and of human MDSCs by S100A9 immunostaining on hematoxylin and eosin (H&E) of tissue sections. Shown is number of areas where MDSCs and CD31 vessels colocalize within tumors inoculated alone (No MDSCs) or with a 25 or 50% MDSC inoculation dose. (D) Levels of suppressive cytokines in serum of mice with tumors alone or inoculated with 25 or 50% MDSC dose. * indicates P < 0.05 vs. same cytokine in other groups. (E) Treatment schema for experiments assessing MDSC dose-dependent immunosuppression. (F) Neuroblastoma antigen GD2-specific CAR-T cells were injected into mice bearing tumor xenograft alone (No MDSCs) or mice bearing xenografts containing 25 or 50% MDSC dose, and tumor volume was followed over time. Control mice received non-CAR modified T cells (no Tx). (G) Treatment schema for experiments assessing effect of MDSC-targeting NKG2D.ζ-modified NK cells on (H) intratumoral MDSCs and (I) tumor volume. ns, not significant; sc, subcutaneously; iv, intravenously.