Fig. 4. Divalent cation binding site of OTR.
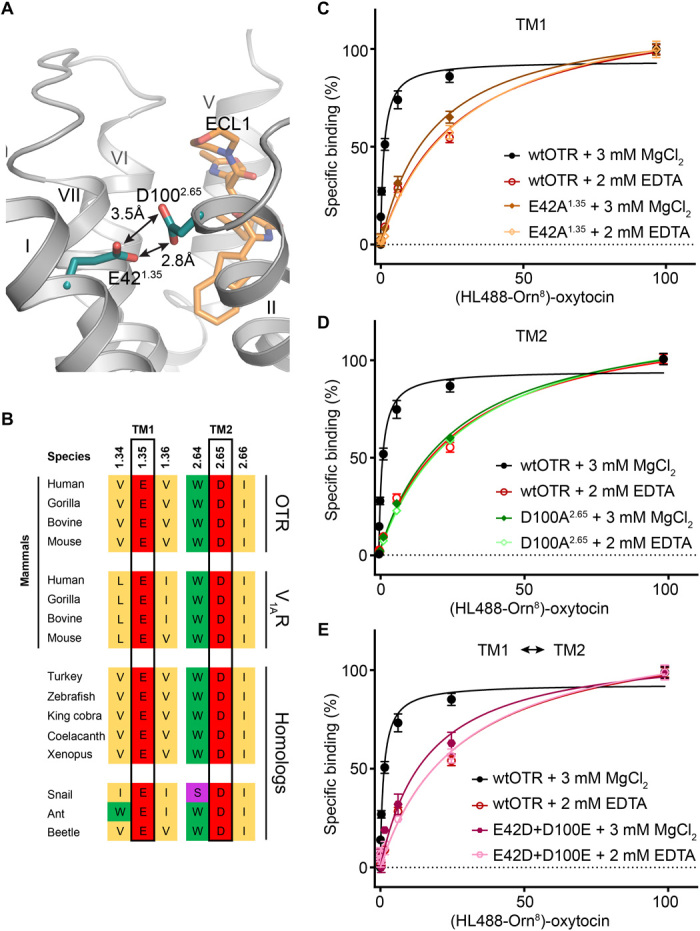
(A) Location of the allosteric divalent cation binding site created by E421.35 and D1002.65 shown in cyan sticks with head group oxygen-oxygen distances indicated by arrows. (B) Amino acid sequence alignment of specific residues comprising the human OTR divalent cation binding site (black boxes). The divalent cation binding site of human OTR is highly conserved in homologs from different taxa including mammals, nonmammalian vertebrates, and invertebrates. Furthermore, the divalent cation binding site of OTR is also conserved in mammalian homologs (human, gorilla, bovine, and mouse) of the closely related V1AR of the oxytocin/vasopressin system. Accession codes are provided in fig. S5A. (C) Specific oxytocin binding to wtOTR and wtOTR with E421.35 of the divalent cation binding site mutated to alanine (E421.35A) in the presence (3 mM MgCl2) or absence (2 mM EDTA) of Mg2+. Data are shown as means ± SEM from three independent experiments performed in triplicates (table S5). (D) As in (C) with the mutant D1002.65A. (E) As in (C) with a double mutant, in which both acidic residues of the divalent cation binding site are interchanged (E421.35D and D1002.65E).
