Abstract
Background
Recently, it has been found that the gut microbiota may affect the development of lung cancer through the “gut‐lung axis.” To investigate this relationship, we performed this study to determine whether the gut microbiota in non‐small‐cell lung cancer (NSCLC) patients is different from that in healthy adults.
Methods
Quantitative PCR (qPCR) was used to detect the expression levels of eight gut butyrate‐producing bacteria in healthy adults and NSCLC patients. We enrolled 30 patients with NSCLC and 30 subjects from 100 healthy adults after matching for age and sex.
Results
Compared to healthy adults, most of the gut butyrate‐producing bacteria in NSCLC patients were significantly decreased; these included Faecalibacterium prausnitzii, Clostridium leptum, Clostridial cluster I, Ruminococcus spp., Clostridial Cluster XIVa, and Roseburia spp. Among the gut butyrate‐producing bacteria, we analyzed Clostridial cluster IV and Eubacterium rectale were not decreased in NSCLC patients.
Conclusions
We conclude that NSCLC patients had gut butyrate‐producing bacteria dysbiosis. Further studies should be performed to investigate the underlying mechanisms of how these specific bacteria affect lung cancer progression and prognosis.
Keywords: butyrate‐producing bacteria, dysbiosis, gut microbiota, gut‐lung axis, non‐small‐cell lung cancer
1. INTRODUCTION
Globally, lung cancer is the most commonly diagnosed cancer (11.6% of the total new cancer cases) and is the leading cause of cancer deaths (18.4% of the total cancer deaths). 1 In China, lung cancer is associated with the highest cancer‐related morbidity and mortality. 2 The incidence is about 40/100 000 per year, while the average global incidence is 31.5/100 000 per year. 1 In addition to smoking, air pollution, occupational carcinogenic factors, ionizing radiation, and genetic factors, there are significant changes in the microbiota in lung cancer patients, suggesting that microbiota dysbiosis may also play an important role in lung cancer pathogenesis. 3 However, most of the lung cancer microbiota studies focus exclusively on the lung microbiota as opposed to other microbiota that may be implicated in lung cancer. 4 , 5 , 6 , 7 , 8 , 9 For example, the oral microbiota is associated with the occurrence and development of lung cancer. 10 , 11 , 12 , 13 This mechanism may be related to several potential processes, including microbiota dysbiosis, genotoxicity and virulence effect, metabolism, inflammation, and immune response. 3
As the most complex microbiota system, the gut microbiota is closely related to innate immunity and adaptive immunity, nutrient absorption, metabolism, tissue development, and inflammatory response. It is well established that gut microbiota influences gastrointestinal cancer. 14 Similarly, extraintestinal cancers are also associated with gut microbiota. 15 In recent years, some studies have reported the relationship between lung cancer and the gut microbiota. Zhang et al found that lung cancer patients had significantly higher levels of Bacteroidetes, Fusobacteria, Cyanobacteria, Spirochaetes, and Lentisphaerae, but dramatically lower levels of Firmicutes and Verrucomicrobia than the healthy participants. 16 The study showed that eight predominant genera were significantly different between the two groups.
Studies have suggested that lung cancer patients not only have different gut microbiota, but more importantly, these microbiota affect the therapeutic prognosis of lung cancer. In 2018, a study reported that primary resistance to immune checkpoint inhibitors (ICIs) can be attributed to abnormal gut microbiota composition. 17 Because Akkermansia muciniphila is often decreased in lung cancer patients, supplementation with Akkermansia muciniphila can improve the ICI response. 17 Another study using the Lewis lung cancer mouse model demonstrated that the commensal microbiota contributes to the anti‐lung cancer response, with probiotic co‐treatment enhancing the anti‐growth and pro‐apoptotic effects of cisplatin. 18
The specific mechanism by which the gut microbiota affects lung cancer is unknown, though some studies indicate that it may be related to immunity. 16 , 17 , 18 One potential mechanism may involve the gut‐lung axis. The existence of a lung‐gut axis has been suggested more recently, and the basis of this axis theory lies in the “gut‐lymph” theory of Samuelson et al. 19 Alterations of this axis have been suggested to result in deleterious consequences, such as pathogen colonization, increased susceptibility to infection, tissue damage, possible development of cancer, and increased mortality. 20 , 21 With this in mind, it is evident that gut microbiota plays a crucial role in homeostasis in hosts, and that its fine‐tuned composition counts for much more than was previously thought.
Therefore, to elucidate this new and very interesting area of research, we conducted a case‐control observational study of NSCLC patients. This study consisted of two groups of patients: Group 1 (NSCLC) and Group 2 (healthy volunteers). Transcript expression in the gut microbiota was evaluated by qPCR, focusing exclusively on the differences in butyrate‐producing bacteria.
2. MATERIAL AND METHODS
2.1. Participant information
This study was approved by the Clinical Research Ethics Committee of First Affiliated Hospital, School of Medicine, Zhejiang University. The study was performed in accordance with the 7th revision of the Declaration of Helsinki (2013), and all participants provided signed, written informed consent. All subjects were ≥18 years old. All the enrolled subjects reported demographic information, including height and weight, sex, age, smoking and drinking habits, lifestyle, and diet. For NSCLC patients, the details also included laboratory tests, tumor pathological type, tumor stage, and tumor site. Exclusion criteria were as follows: history of radiotherapy or surgery; diagnosis of a malignant tumor (except lung cancer); presence of cardiovascular disease (myocardial infarction or stroke); presence of diabetes, hypertension, dyslipidemia, or dementia; activities of daily living (ADL) score <100 points; history of depression; use of probiotics, prebiotics, synbiotics, or antibiotics in the past 4 weeks before enrollment; and history of gastrointestinal surgery. From September 2014 to August 2017, 41 patients with pathological diagnosis of NSCLC were enrolled in the Geriatric ward at the First Affiliated Hospital of Zhejiang University School of Medicine, among which 30 patients were eligible. One hundred healthy adults were enrolled during the same period, and 30 participants were selected for the control group after matching for age and sex.
2.2. Fecal sample collection, DNA extraction, and PCR
Fresh stool samples from NSCLC patients and healthy controls were collected in anaerobic bags. Samples were divided into five aliquots of 200 mg within 30 minutes after sampling and were immediately stored at −80°C.
2.3. Detection of butyrate‐producing bacteria in the gut by qPCR
All oligonucleotide primers were synthesized by Takara (see Table 1). qPCR was performed using a ViiA 7 real‐time PCR system (Applied Biosystems). The amplification was performed using a commercially available kit (TAKARA SYBR Premix EX TaqTM; kit Code: DRR820A): 10 µL SYBR Green PCR premix (Applied Biosystems; Code: 4309155), 0.8 µL of each primer, 2 µL of original template DNA, distilled water to make the final volume to 20 µL. Each reaction was repeated twice, and the error was controlled within 0.5. The amplification temperature and time were as follows: pre‐denaturation at 95°C for 3 minutes, followed by 40 cycles of denaturation at 95°C for 30 seconds, annealing at 72°C for 40 seconds, extension at 72°C for 30 seconds, and a final extension at 72°C for 5 minutes. Fluorescence was measured at 80°C for 10 seconds after the extension step of each cycle to avoid interference from primer dimers, spurious priming, or secondary structure. A final extension step was performed for 5 minutes at 72°C. Following amplification, melting temperature analysis of PCR products was performed to determine the specificity of the PCR. The melting curves were obtained by heating at from 65°C to 95°C at a rate of 0.1°C/s, with continuous fluorescence measurement. The copy number of rDNA operons of these butyrate‐producing bacteria in the gut in crude DNA templates was determined by comparing serially diluted plasmid DNA standards run on the same plate. The annealing temperature and sequences for each primer were as previously described and are presented in Table 1.22, 23, 24, 25, 26, 27, 28, 29
Table 1.
Primer sequences and annealing temperatures in this study
| Primers | Sequence (5′‐3′) | Annealing temperature (°C) |
|---|---|---|
| Faecalibacterium prausnitzii |
GATGGCCTCGCGTCCGATTAG CCGAAGACCTTCTTCCTCC |
58 22 |
| Clostridium leptum |
TTAACACAATAAGTWATCCACCTGG ACCTTCCTCCGTTTTGTCAAC |
60 23 , 24 |
| Clostridial cluster I |
ATGCAAGTCGAGCGAKG TATGCGGTATTAATCTYCCTTT |
60 25 |
| Eubacterium rectale |
AAG GGAAGCAAAGCTGTGAA TCGGTTAGGTCACTGGCTTC |
61 26 |
| Clostridial cluster IV |
CCTCTTGACCGGCGTGT CAGGTAGAGCTGGGCACTCTAGG |
58 25 |
| Ruminococcus spp. |
GGCGGCYTRCTGGGCTTT CCAGGTGGATWACTTATTGTGTTAA |
60 27 |
| Clostridial cluster XIVa |
CGGTACCTGACTAAGAAGC AGTTTYATTCTTGCGAACG |
55 28 |
| Roseburia spp. |
GCGGTRCGGCAAGTCTGA CCTCCGACACTCTAGTMCGA |
60 29 |
2.4. Statistical analysis
All continuous variables were expressed as mean ± standard deviation (SD). The comparison between two groups was performed using the Student's t test for independent samples. The categorical variables were tested by chi‐squared test. SPSS version 22.0 (SPSS) was used for statistical analysis. Moreover, we evaluated the Spearman's rank correlation between systemic inflammatory markers and gut butyrate‐producing bacteria using RStudio version 3.6.1 (RStudio). A value of P < .05 was considered statistically significant.
3. RESULTS
3.1. Participant characteristics
A total of 30 patients (20 males and 10 females) comprised the NSCLC group, and 30 individuals (16 males and 14 females) were included in the healthy control group. There were no significant differences in age, sex, BMI, or smoking, and drinking habits between the two groups. In the NSCLC group, 26 patients (86.7%) had adenocarcinoma and 4 patients (13.3%) had squamous cell carcinoma; 24 patients (80%) had peripheral lung cancer, and 6 patients (20%) had central lung cancer; and most of the NSCLC patients (20 patients, 66.7%) belonged to the tumor stage Group I (Table 2).
Table 2.
Characteristics of the NSCLC and healthy groups
| Healthy group | NSCLC group | P value | |
|---|---|---|---|
| n | 30 | 30 | |
| Sex (male/female) | 16/14 | 20/10 | .292 |
| Age (y) | 67.4 ± 6.8 | 66.0 ± 7.3 | .423 |
| Smoking (%) | 26.7 | 46.7 | .108 |
| Drinking (%) | 23.3 | 26.7 | .766 |
| BMI (Kg/m2) | 23.6 ± 2.7 | 22.4 ± 2.7 | .104 |
| Tumor stage | |||
| I | 20 | ||
| II | 2 | ||
| III | 5 | ||
| IV | 3 | ||
| Pathological classification | |||
| Adenocarcinoma | 26 | ||
| Squamous cell cancer | 4 | ||
| Tumor site | |||
| Peripheral lung cancer | 24 | ||
| Central lung cancer | 6 | ||
The continuous variables are listed as mean ± SD.
Abbreviation: BMI, body mass index.
3.2. Detection of butyrate‐producing bacteria in the gut by qPCR
Gut butyrate‐producing bacteria in the two groups were measured by qPCR. First, we performed logarithmic processing of these data, and they were expressed as log10 gene copies in 1 μg fecal DNA; this was followed by comparison using the t test. The following bacteria were significantly lower in the NSCLC group compared to that in the control group: Faecalibacterium prausnitzii (6.7 ± 1.0 vs 7.5 ± 1.1, P = .006), Clostridium leptum (7.1 ± 1.0 vs 8.0 ± 0.9, P = .001), Clostridial cluster I (5.1 ± 1.2 vs 5.9 ± 0.8, P = .002), Ruminococcus spp. (5.7 ± 1.0 vs 6.6 ± 1.0, P = .001), Clostridial cluster XIVa (6.7 ± 1.6 vs 8.0 ± 0.8, P < .0001), and Roseburia spp. (5.9 ± 1.1 vs 6.6 ± 1.4, P = .035). However, there were no significant differences in Clostridial cluster IV (3.0 ± 0.9 vs 3.0 ± 1.3, P = .79) and Eubacterium rectale (5.4 ± 1.4 vs 6.2 ± 1.5, P = .37; Table 3).
Table 3.
Comparison of the logarithmic counts (log10 copies/µg) of some gut butyrate‐producing bacteria between the two groups
|
Healthy group (n = 30) |
NSCLC group (n = 30) |
P values | |
|---|---|---|---|
| Faecalibacterium prausnitzii | 7.5 ± 1.1 | 6.7 ± 1.0 | .006 |
| Clostridium leptum | 8.0 ± 0.9 | 7.1 ± 1.0 | .001 |
| Clostridial cluster I | 5.9 ± 0.8 | 5.1 ± 1.2 | .002 |
| Eubacterium rectale | 6.2 ± 1.5 | 5.4 ± 1.4 | .37 |
| Clostridial cluster IV | 3.0 ± 1.3 | 3.0 ± 0.9 | .79 |
| Ruminococcus spp. | 6.6 ± 1.0 | 5.7 ± 1.0 | .001 |
| Clostridial cluster XIVa | 8.0 ± 0.8 | 6.7 ± 1.6 | <.0001 |
| Roseburia spp. | 6.6 ± 1.4 | 5.9 ± 1.1 | .035 |
The continuous variables are listed as mean ± SD.
Abbreviation: NSCLC, non‐small‐cell lung cancer.
3.3. Correlation between systemic inflammatory markers and butyrate‐producing bacteria
Systemic inflammation‐related markers, including white blood cells (WBC), neutrophils, lymphocytes, monocytes, neutrophil‐to‐lymphocyte ratio (NLR), platelet to‐lymphocyte ratio (PLR), lymphocyte‐to‐monocyte ratio (LMR), and C‐reactive protein, are potentially independent prognostic factors in lung cancer survival. 16 With the exception of Roseburia spp. and Clostridial cluster IV, which were negatively correlated with monocytes and WBCs, respectively, all the other tested bacteria were not correlated with the inflammation‐related markers (Figure 1).
Figure 1.
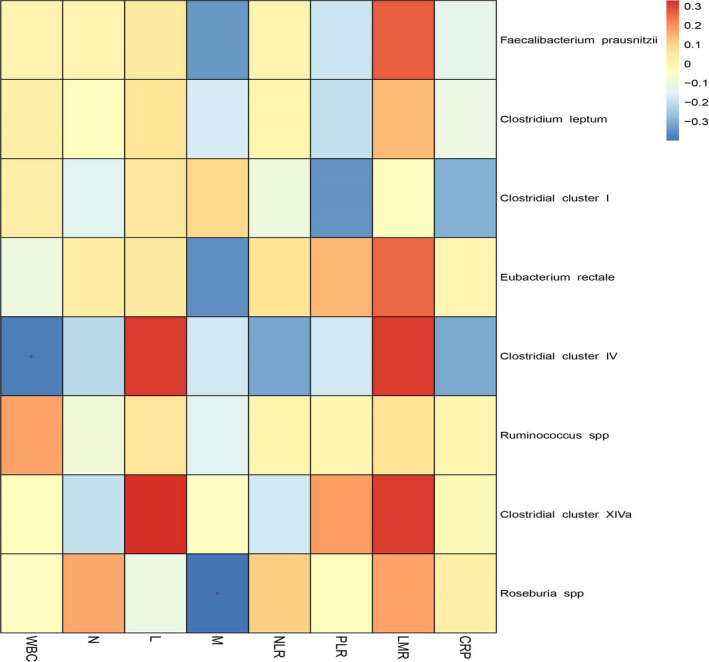
Correlations between systemic inflammatory indicators and butyrate‐producing bacteria. The Spearman's rank correlation was used to evaluate the statistical importance between systemic inflammatory indicators and the relative abundance of eight butyrate‐producing bacteria and was represented by color ranging from blue (negative correlation) to red (positive correlation). CRP, C‐reactive protein; L, lymphocytes; LMR, lymphocyte‐monocyte ratio; M, monocytes; N, neutrophils; NLR, neutrophil‐lymphocyte ratio; PLR, platelet‐lymphocyte ratio; WBC, white blood cells; + P < .05
4. DISCUSSION
In recent years, studies on the roles of the gut microbiota in extra‐gastrointestinal tumors have been increasing, 15 , 30 and the relationship between lung cancer and the gut microbiota has also been investigated. 16 , 17 , 18 Zhang et al first disclosed the characteristics of the gut microbiota in lung cancer patients. 16 The present study focused exclusively on determining the differences among eight gut butyrate‐producing bacteria, which are the most abundant butyrate‐producing bacterial species in the gut, using qPCR. 31 , 32 In addition, these bacteria play an important role in maintaining gut homeostasis by improving gut barrier functions and exerting anti‐inflammatory and immunomodulatory effects. 33 There is considerable literature evaluating these eight butyrate‐producing bacteria using the PCR technology, 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 and thus, this approach is relatively established, ensuring reliability of the results.
We found that compared to healthy individuals, except for Clostridial cluster IV and Eubacterium rectale, all the other tested butyrate‐producing bacteria, including Faecalibacterium prausnitzii, Clostridium leptum, Clostridial cluster I, Ruminococcus spp., Clostridial cluster XIVa, and Roseburia spp., were significantly decreased, indicating that there is significant dysbiosis of butyrate‐producing bacteria in NSCLC patients. Several studies 16 , 34 have demonstrated the presence of gut microbiota dysbiosis in patients with lung cancer, which is consistent with our findings. However, given the differences in the gut microbiota of interest, sampling locations, participant age, and determination methods between our study and previous studies, the specific gut bacterial species in our findings were not the same as those in the other two studies. 16 , 34 For example, Zhuang et al 34 found that patients with lung cancer have elevated levels of Enterococcus. Further, Zhang et al 16 found that lung cancer patients have higher levels of Bacteroides, Veillonella, and Fusobacterium but lower levels of Escherichia‐Shigella, Kluyvera, Faecalibacterium, Enterobacter, and Dialister.
Butyrate‐producing bacteria are a type of gut probiotic, which produce butyric acid as their main metabolite. These bacteria exist in the human intestine and can inhibit the growth of harmful gastrointestinal bacteria, promote nutrient absorption, improve intestinal function, and can be beneficial for the overall human health. Butyrate‐producing bacteria are also found in the human colon. The most important species appear to be Faecalibacterium prausnitzii, Eubacterium rectale, and Roseburia intestinalis belonging to clostridial clusters IV and XIVa, which belong to the Firmicutes phylum. 35 , 36 , 37
Butyrate‐producing bacteria have received much attention in the past few years because they contribute to intestinal homeostasis by maintaining the intestinal barrier function and exerting immunomodulatory and anti‐inflammatory effects. 32 , 33 Studies have shown that butyrate‐producing bacteria are negatively related to irritable bowel disease and colorectal cancer, 38 , 39 , 40 as butyric acid is typically decreased in such patients. The abundance of butyrate‐producing bacteria is also lower in patients with metabolic diseases. 41
As an important product of butyrate‐producing bacteria, butyric acid is a major short chain fatty acid (SCFA) preferentially used as an energy source by the gut mucosa. 31 Interestingly, sodium butyrate has shown potent anti‐cancer activity as a potential histone deacetylase inhibitor. 42 Particularly in colorectal cancer, sodium butyrate can induce apoptosis of tumor cells. 43 It has also been found that sodium butyrate can inhibit the growth of lung cancer cells. In vitro experiments have shown that sodium butyrate increases the expression of P‐glycoprotein in lung cancer cells by upregulation of signal transducer and activator of transcription 3 (STAT3) and stabilization of the ATP binding cassette subfamily B member 1 (ABCB1) mRNA. 44
To explore the possible mechanisms underlying the decrease in gut butyrate‐producing bacteria, we analyzed correlations between systemic inflammatory markers and gut butyrate‐producing bacteria and found that most butyrate‐producing bacteria were not significantly related to inflammatory markers. We reached a different conclusion from those made in previous studies, which generally indicated significant relationships between butyrate‐producing bacteria and inflammatory markers. 45 , 46 , 47 Such other studies 48 , 49 , 50 , 51 showed that NLR, LMR, and PLR are associated with poor prognosis in patients with NSCLC. In our study, only two of the eight bacteria were negatively related to WBCs and monocytes, and no bacteria were significantly associated with the other systemic inflammatory markers (such as NLR, PLR, and LMR). Therefore, we could not conclude that butyric acid bacteria influence systemic inflammation. Our findings might be related to the small number of cases or the lack of a causal relationship between systemic inflammatory markers and gut butyrate‐producing bacteria in NSCLC.
Recently, many studies have begun to address the relationship between gut microbiota and the lung, which has been referred to as the “gut‐lung axis”. 19 This theory suggests that the gut microbiota can affect the lungs through local and systemic immune responses. As early as 2015, there were related research studies focused on tumor and gut microbiota. 20 , 21 In recent years, clinical studies of lung cancer have also confirmed that the gut‐lung axis was affected by immunization. In 2018, there were three reports of a relationship between the gut microbiota and ICIs (PD‐1/PD‐L1). 17 , 52 , 53 Combining these highly significant fields of research, we can conclude that gut microbiota is closely related to the immune system, which has a great effect on the efficacy of tumor immunotherapy. Different gut microbiota play different roles in immunity; some “good microbiota” can significantly enhance the efficacy of immunotherapy, whereas “bad microbiota” will not. One of these studies was based on NSCLC patients, which found that the gut microbiota is closely related to the ICI efficacy. 17 Another study showed that some Chinese lung cancer patients with high diversity of gut microbiota showed significantly better response to nivolumab immunotherapy. In terms of progression‐free (PR) survival, patients with high gut microbiota diversity can reach 209 days, while patients with low diversity show a PR survival rate of only 52 days. 54
There are several limitations to our study that should be acknowledged. First, the sample size was small, which might influence the outcome of this study. Second, we only assessed the relationship between systemic inflammation indicators and gut butyrate‐producing bacteria in NSCLC and did not explore whether the gut‐lung axis plays an important role in NSCLC progression. Moreover, we did not further measure the inflammatory factors, such as interleukin‐12, interleukin‐17, and cytotoxic T lymphocyte antigen‐4, which might be related to lung cancer, based on results of a previous study. 16 Finally, we did not perform animal experiments to further validate our findings.
5. CONCLUSIONS
In conclusion, our study confirms a significant correlation between the gut microbiota and lung cancer. Whether lung cancer patients carry a different microbiota or whether the differences in the gut microbiota affect the efficacy of immune‐based cancer therapeutics, the gut‐lung relationship may be related to the immune system. Although previous studies have found that butyrate‐producing bacteria play a certain role in the occurrence and development of tumors, and this is supported by the present study, further research is needed to verify its role in lung cancer. This study was a cross‐sectional study, as we did not specifically evaluate the mechanism between gut bacteria and tumor development. In summary, we found that dysbiosis is implicated in NSCLC patients, who have lower levels of gut butyrate‐producing bacteria. Further, we found a negative correlation between systemic inflammation markers and gut butyrate‐producing bacteria in NSCLC patients. Although we speculate that butyrate‐producing bacteria may affect lung cancer through the gut‐lung axis, the related mechanism is still unknown. Future studies are warranted to investigate the specific mechanism(s) by which the gut microbiota influences the development of lung tumor.
Gui Q, Li H, Wang A, et al. The association between gut butyrate‐producing bacteria and non‐small‐cell lung cancer. J Clin Lab Anal. 2020;34:e23318 10.1002/jcla.23318
Funding information
The present study was supported by the Zhejiang Provincial Medical and Health Research Program (2017‐KY1‐002‐024), the National Clinical Key Specialty Construction Project of Geriatrics, the Key Disciplines Construction Plan of Zhejiang Province Traditional Chinese Medicine (2017‐XK‐A31), and the National Key Research and Development Program of China (2018YFC2000301, 2018YFC2000501).
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Fitzmaurice C, Akinyemiju TF, Al Lami FH, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability‐adjusted life‐years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4(11):1553‐1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mao Q, Jiang F, Yin R, et al. Interplay between the lung microbiome and lung cancer. Cancer Lett. 2018;415:40‐48. [DOI] [PubMed] [Google Scholar]
- 4. Laroumagne S, Salinas‐Pineda A, Hermant C, et al. Incidence and characteristics of bronchial colonisation in patient with lung cancer: a retrospective study of 388 cases. Rev Mal Respir. 2011;28(3):328‐335. [DOI] [PubMed] [Google Scholar]
- 5. Hosgood HD 3rd, Sapkota AR, Rothman N, et al. The potential role of lung microbiota in lung cancer attributed to household coal burning exposures. Environ Mol Mutagen. 2014;55(8):643‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cameron SJS, Lewis KE, Huws SA, et al. A pilot study using metagenomic sequencing of the sputum microbiome suggests potential bacterial biomarkers for lung cancer. PLoS ONE. 2017;12(5):e0177062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee SH, Sung JY, Yong D, et al. Characterization of microbiome in bronchoalveolar lavage fluid of patients with lung cancer comparing with benign mass like lesions. Lung Cancer. 2016;102:89‐95. [DOI] [PubMed] [Google Scholar]
- 8. Yu G, Gail MH, Consonni D, et al. Characterizing human lung tissue microbiota and its relationship to epidemiological and clinical features. Genome Biol. 2016;17(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu HX, Tao LL, Zhang J, et al. Difference of lower airway microbiome in bilateral protected specimen brush between lung cancer patients with unilateral lobar masses and control subjects. Int J Cancer. 2018;142(4):769‐778. [DOI] [PubMed] [Google Scholar]
- 10. Vogtmann E, Goedert JJ. Epidemiologic studies of the human microbiome and cancer. Br J Cancer. 2016;114(3):237‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zeng XT, Xia LY, Zhang YG, Li S, Leng WD, Kwong JS. Periodontal disease and incident lung cancer risk: a meta‐analysis of cohort studies. J Periodontol. 2016;87(10):1158‐1164. [DOI] [PubMed] [Google Scholar]
- 12. Michaud DS, Kelsey KT, Papathanasiou E, Genco CA, Giovannucci E. Periodontal disease and risk of all cancers among male never smokers: an updated analysis of the health professionals follow‐up study. Ann Oncol. 2016;27(5):941‐947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yan X, Yang M, Liu J, et al. Discovery and validation of potential bacterial biomarkers for lung cancer. Am J Cancer Res. 2015;5(10):3111‐3122. [PMC free article] [PubMed] [Google Scholar]
- 14. Lam SY, Yu J, Wong SH, Peppelenbosch MP, Fuhler GM. The gastrointestinal microbiota and its role in oncogenesis. Best Pract Res Clin Gastroenterol. 2017;31(6):607‐618. [DOI] [PubMed] [Google Scholar]
- 15. Fernandez MF, Reina‐Perez I, Astorga JM, Rodriguez‐Carrillo A, Plaza‐Diaz J, Fontana L. Breast Cancer and Its Relationship with the Microbiota. Int J Environ Res Public Health. 2018;15(8):1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang WQ, Zhao SK, Luo JW, et al. Alterations of fecal bacterial communities in patients with lung cancer. Am J Transl Res. 2018;10(10):3171‐3185. [PMC free article] [PubMed] [Google Scholar]
- 17. Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD‐1‐based immunotherapy against epithelial tumors. Science (New York, NY). 2018;359(6371):91‐97. [DOI] [PubMed] [Google Scholar]
- 18. Gui QF, Lu HF, Zhang CX, Xu ZR, Yang YH. Well‐balanced commensal microbiota contributes to anti‐cancer response in a lung cancer mouse model. Genet Mol Res. 2015;14(2):5642‐5651. [DOI] [PubMed] [Google Scholar]
- 19. Samuelson DR, Welsh DA, Shellito JE. Regulation of lung immunity and host defense by the intestinal microbiota. Front Microbiol. 2015;6:1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122(1):107‐118. [DOI] [PubMed] [Google Scholar]
- 21. Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science (New York, NY). 2012;336(6086):1268‐1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang RF, Cao WW, Cerniglia CE. PCR detection and quantitation of predominant anaerobic bacteria in human and animal fecal samples. Appl Environ Microbiol. 1996;62(4):1242‐1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lay C, Sutren M, Rochet V, Saunier K, Dore J, Rigottier‐Gois L. Design and validation of 16S rRNA probes to enumerate members of the Clostridium leptum subgroup in human faecal microbiota. Environ Microbiol. 2005;7(7):933‐946. [DOI] [PubMed] [Google Scholar]
- 24. Sghir A, Gramet G, Suau A, Rochet V, Pochart P, Dore J. Quantification of bacterial groups within human fecal flora by oligonucleotide probe hybridization. Appl Environ Microbiol. 2000;66(5):2263‐2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Song Y, Liu C, Finegold SM. Real‐time PCR quantitation of clostridia in feces of autistic children. Appl Environ Microbiol. 2004;70(11):6459‐6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Balamurugan R, Janardhan HP, George S, Raghava MV, Muliyil J, Ramakrishna BS. Molecular studies of fecal anaerobic commensal bacteria in acute diarrhea in children. J Pediatr Gastroenterol Nutr. 2008;46(5):514‐519. [DOI] [PubMed] [Google Scholar]
- 27. Harmsen HJ, Raangs GC, He T, Degener JE, Welling GW. Extensive set of 16S rRNA‐based probes for detection of bacteria in human feces. Appl Environ Microbiol. 2002;68(6):2982‐2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsuki T, Watanabe K, Fujimoto J, et al. Development of 16S rRNA‐gene‐targeted group‐specific primers for the detection and identification of predominant bacteria in human feces. Appl Environ Microbiol. 2002;68(11):5445‐5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walker AW, Duncan SH, McWilliam Leitch EC, Child MW, Flint HJ. pH and peptide supply can radically alter bacterial populations and short‐chain fatty acid ratios within microbial communities from the human colon. Appl Environ Microbiol. 2005;71(7):3692‐3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raza MH, Gul K, Arshad A, et al. Microbiota in cancer development and treatment. J Cancer Res Clin Oncol. 2019;145(1):49‐63. [DOI] [PubMed] [Google Scholar]
- 31. Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19(1):29‐41. [DOI] [PubMed] [Google Scholar]
- 32. Riviere A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and butyrate‐producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol. 2016;7:979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Velasquez‐Manoff M. Gut microbiome: the peacekeepers. Nature. 2015;518(7540):S3‐11. [DOI] [PubMed] [Google Scholar]
- 34. Zhuang H, Cheng L, Wang Y, et al. Dysbiosis of the gut microbiome in lung cancer. Front Cell Infect Microbiol. 2019;9:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barcenilla A, Pryde SE, Martin JC, et al. Phylogenetic relationships of butyrate‐producing bacteria from the human gut. Appl Environ Microbiol. 2000;66(4):1654‐1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate‐producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 37. De Vuyst L, Leroy F. Cross‐feeding between bifidobacteria and butyrate‐producing colon bacteria explains bifdobacterial competitiveness, butyrate production, and gas production. Int J Food Microbiol. 2011;149(1):73‐80. [DOI] [PubMed] [Google Scholar]
- 38. Gevers D, Kugathasan S, Denson LA, et al. The treatment‐naive microbiome in new‐onset Crohn's disease. Cell Host Microbe. 2014;15(3):382‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nemoto H, Kataoka K, Ishikawa H, et al. Reduced diversity and imbalance of fecal microbiota in patients with ulcerative colitis. Dig Dis Sci. 2012;57(11):2955‐2964. [DOI] [PubMed] [Google Scholar]
- 40. Wu N, Yang X, Zhang R, et al. Dysbiosis signature of fecal microbiota in colorectal cancer patients. Microb Ecol. 2013;66(2):462‐470. [DOI] [PubMed] [Google Scholar]
- 41. Brahe LK, Astrup A, Larsen LH. Is butyrate the link between diet, intestinal microbiota and obesity‐related metabolic diseases? Obes Rev. 2013;14(12):950‐959. [DOI] [PubMed] [Google Scholar]
- 42. Chavan AV, Somani RR. HDAC inhibitors ‐ new generation of target specific treatment. Mini Rev Med Chem. 2010;10(13):1263‐1276. [DOI] [PubMed] [Google Scholar]
- 43. Wu X, Wu Y, He L, Wu L, Wang X, Liu Z. Effects of the intestinal microbial metabolite butyrate on the development of colorectal cancer. J Cancer. 2018;9(14):2510‐2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhao L, Bin S, He HL, et al. Sodium butyrate increases P‐gp expression in lung cancer by upregulation of STAT3 and mRNA stabilization of ABCB1. Anticancer Drugs. 2018;29(3):227‐233. [DOI] [PubMed] [Google Scholar]
- 45. Sitkin S, Pokrotnieks J. Clinical potential of anti‐inflammatory effects of faecalibacterium prausnitzii and butyrate in inflammatory bowel disease. Inflamm Bowel Dis. 2019;25(4):e40‐e41. [DOI] [PubMed] [Google Scholar]
- 46. Li YN, Huang F, Liu L, Qiao HM, Li Y, Cheng HJ. Effect of oral feeding with Clostridium leptum on regulatory T‐cell responses and allergic airway inflammation in mice. Ann Allergy Asthma Immunol. 2012;109(3):201‐207. [DOI] [PubMed] [Google Scholar]
- 47. Li YN, Huang F, Cheng HJ, Li SY, Liu L, Wang LY. Intestine‐derived Clostridium leptum induces murine tolerogenic dendritic cells and regulatory T cells in vitro. Human Immunol. 2014;75(12):1232‐1238. [DOI] [PubMed] [Google Scholar]
- 48. Yilmaz U, Ozdemir O, Batum O, Ermin S. The prognostic role of neutrophil‐lymphocyte ratio and platelet‐lymphocyte ratio in patients with stage III non‐small cell lung cancer treated with concurrent chemoradiotherapy. Indian J Cancer. 2018;55(3):276‐281. [DOI] [PubMed] [Google Scholar]
- 49. Scilla KA, Bentzen SM, Lam VK, et al. Neutrophil‐lymphocyte ratio is a prognostic marker in patients with locally advanced (Stage IIIA and IIIB) non‐small cell lung cancer treated with combined modality therapy. Oncologist. 2017;22(6):737‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kos M, Hocazade C, Kos FT, et al. Prognostic role of pretreatment platelet/lymphocyte ratio in patients with non‐small cell lung cancer. Wien Klin Wochenschr. 2016;128(17–18):635‐640. [DOI] [PubMed] [Google Scholar]
- 51. Hu P, Shen H, Wang G, Zhang P, Liu Q, Du J. Prognostic significance of systemic inflammation‐based lymphocyte‐ monocyte ratio in patients with lung cancer: based on a large cohort study. PLoS ONE. 2014;9(9):e108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti‐PD‐1 immunotherapy in melanoma patients. Science (New York, NY). 2018;359(6371):97‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti‐PD‐1 efficacy in metastatic melanoma patients. Science (New York, NY). 2018;359(6371):104‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jin Y, Dong H, Xia L, et al. The diversity of gut microbiome is associated with favorable responses to anti‐programmed death 1 immunotherapy in chinese patients with NSCLC. J Thorac Oncol. 2019;14(8):1378‐1389. [DOI] [PubMed] [Google Scholar]


