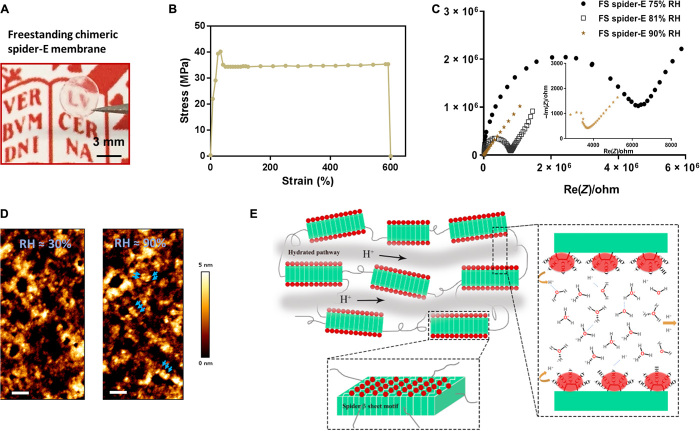
Fig. 4. Bulk freestanding chimeric spider-E membrane with extraordinary proton transfer properties.
(A) A digital photograph illustrates the dimensions and transparency of the membrane. The protein membrane is clamped with a fine tweezer. Photo credit: Chao Ma, University of Groningen. (B) Mechanical characterization of the freestanding (FS) protein membrane, showing a typical tensile stretching curve. (C) Nyquist plot illustrating the conductance behavior of the FS spider-E membrane under different RHs. The film shows best proton translocation properties at 90% RH. (D) AFM characterization of the FS spider-E membrane under ~30 and ~90% RH conditions. Scale bars, 100 nm. Blue arrows point at distinguishable nanostructures. (E) Proposed mechanism of proton transport in the spider-E membrane at RH = 90%. The protons hop between water molecules nanoconfined in the hydrated network of nanodomains formed by spider β sheet motifs (in green). The glutamic acid residues in the chimeric nanostructures present carboxylic groups (in red) on the surface, providing the protons and coordinating water molecules.