Fig. 1. Degradation of the IDP tau by the 20S proteasome.
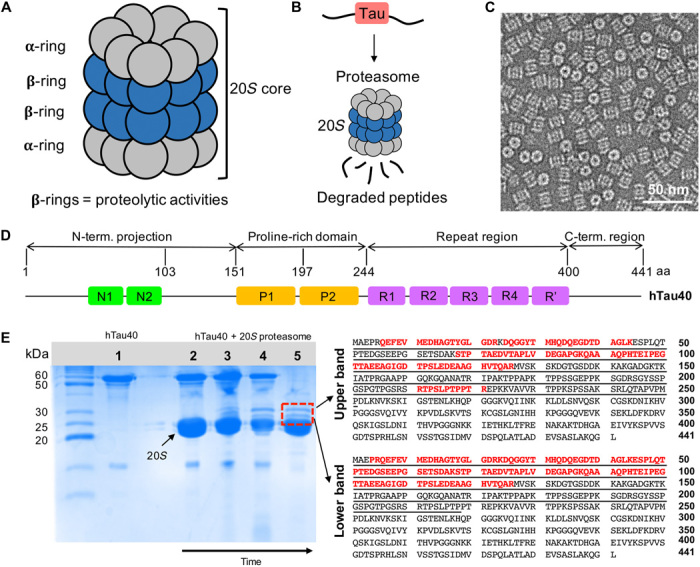
(A) Schematic representation depicting the architecture of the 20S proteasome (20S) comprising 28 subunits arranged in four heptameric rings (α7β7β7α7). (B) The proteolytic active sites of the 20S proteasome are located in its interior, thus enabling degradation of hTau40 into short peptides once it has entered the 20S core. (C) Negatively stained EM micrograph of the 20S proteasome. (D) Domain organization of full-length hTau40 composed of 441 amino acids (aa) (UniProt ID 10636-8). N1 and N2 are the two inserts in the N-terminal projection domain, P1 and P2 correspond to the two proline-rich regions, and R1 to R′ are five pseudo-repeats. (E) (Left) SDS-PAGE gel showing hTau40 (1) and the degradation of (2 to 5) hTau40 by the 20S proteasome over time. The samples were incubated at 37°C for 30 min (2), 90 min (3), and 150 min (4) and were subsequently put at 4°C for additional 48 hours (5). After 48 hours, two well-resolved bands at ~28 and ~30 kDa (red lined box) appeared. (Right) The amino acid sequences of the upper (~30 kDa) and lower bands were identified with in-gel analysis and marked in red. Both intermediates correspond to the N-terminal domain of hTau40.
