Fig. 5. Loss of ARID1A promotes adrenergic-to-mesenchymal transition and increases cisplatin resistance in NGP cells.
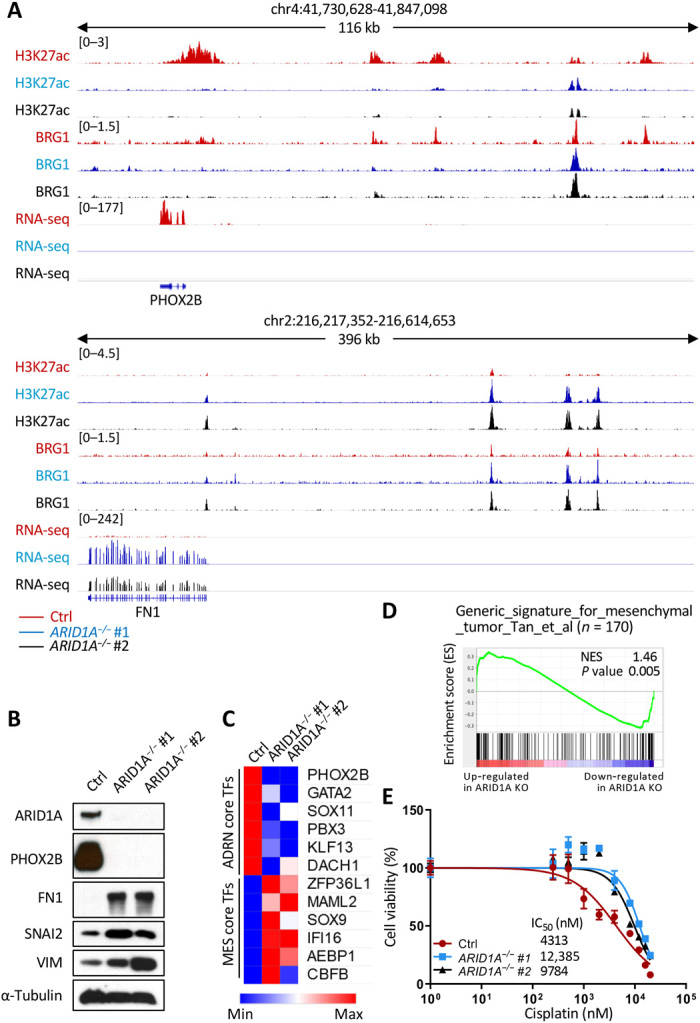
(A) H3K27ac and BRG1 ChIP-seq tracks and RNA-seq tracks at PHOX2B and FN1 loci in control (red), ARID1A−/− #1 (blue), and ARID1A−/− #2 (black) NGP cells. (B) Western blot analysis of ARID1A, an adrenergic maker (PHOX2B), and three mesenchymal markers (FN1, SNAI2, and VIM) in control (Ctrl), ARID1A−/− #1, and ARID1A−/− #2 NGP cells. α-Tubulin was used as a loading control. (C) mRNA expression (RPKM of RNA-seq) of core regulatory circuitries of transcription factors specific for adrenergic (ADRN core TFs) or mesenchymal (MES core TFs) cells in control (Ctrl), ARID1A−/− #1, and ARID1A−/− #2 NGP cells. (D) GSEA to determine the enrichment of a generic gene signature for mesenchymal tumor in ARID1A mutant NGP cells. Genes are ranked by score and plotted along the x axis as vertical black bars. NES, normalized enrichment score; KO, knockout. (E) Cell viability [% relative to N,N′-dimethylformamide (DMF)–treated cells, y value] of control (red), ARID1A−/− #1 (blue), and ARID1A−/− #2 (black). NGP cells were measured after treatment with various concentrations of cisplatin (0 to 20,000 nM, x value) for 72 hours, and half-maximal inhibitory concentration (IC50) values are indicated. Values are means ± SD of triplicate experiments.
