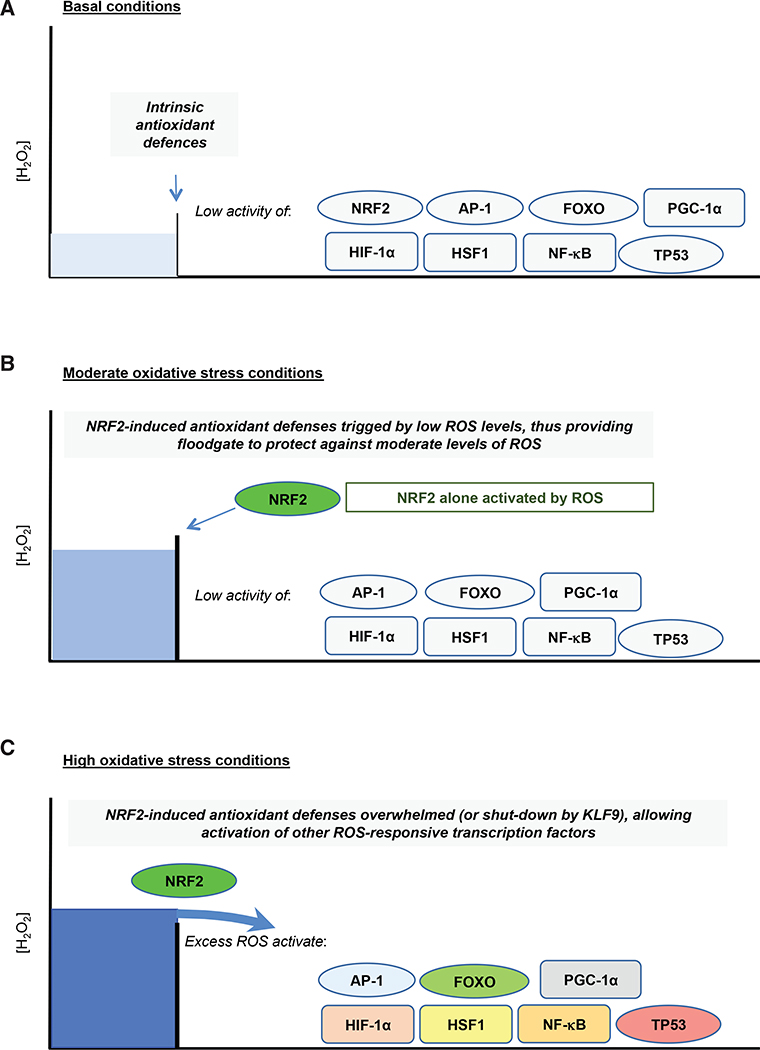
Figure 2. NRF2 Provides an Inducible Floodgate Defense against Oxidative Stress.
Under non-stressed basal conditions (A), cellular redox homeostasis is maintained by constitutive expression of a battery of antioxidant genes. However, when exposed to acute oxidative stress (B), cells adapt to the increase in ROS levels by inducing genes encoding detoxification, GSH- and TXN-dependent antioxidants, and NADPH-generating enzymes that are regulated by NRF2. Should the capacity of the antioxidant systems that are induced by NRF2 become saturated and therefore insufficient to counter additional oxidative stress (C), or prolonged oxidative stress causes activation of KLF9 and downregulation of NRF2, the excess levels of ROS that are not countered by the NRF2-directed defences then trigger additional redox switches that activate other members of the antioxidant transcription factor network. When NRF2-orchestrated defences become saturated, activation of other members of the antioxidant transcription factor network may occur simultaneously, or they may be activated in a stratified manner with each transcription factor being activated at a distinct ROS threshold, which results in various cellular responses, including metabolic reprogramming, damage repair, cell-cycle arrest, senescence, and apoptosis.