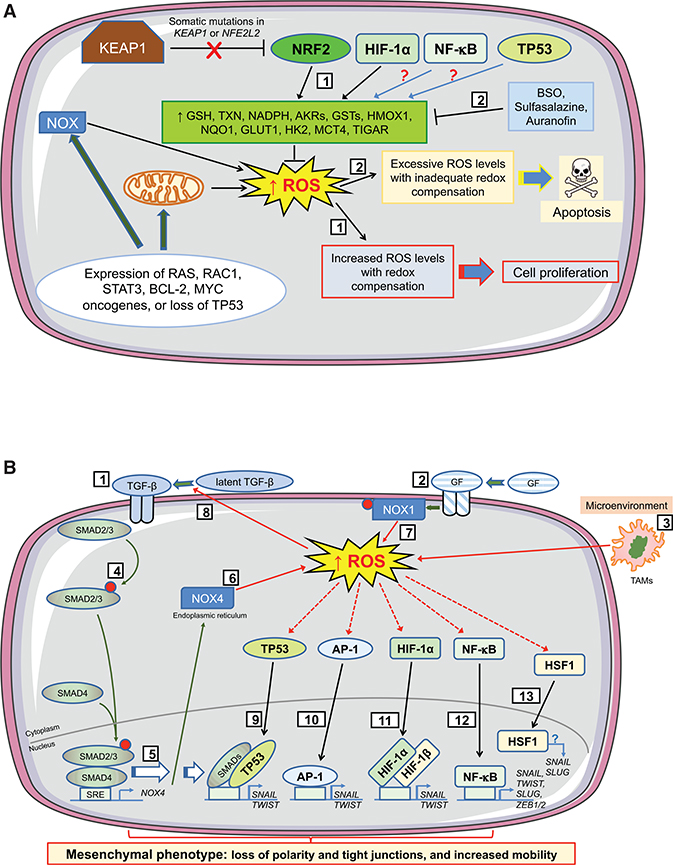
Figure 5. Influence of Oxidative Stress on Cell Fate during Early Stages of Tumorigenesis.
The cartoons depict how levels of ROS stimulate proliferation or apoptosis in preneoplastic cells during initiation of tumorigenesis, and how ROS support EMT during progression of tumorigenesis by altering TGF-β signaling and by activation of antioxidant transcription factors that control expression of EMT-TFs. During the earliest stages of tumorigenesis (A), activation of oncogenes, coupled with higher metabolic demands, results in an increase in intracellular ROS levels in early neoplastic lesions/adenomas. To benefit from the proliferative advantages associated with the increase in ROS, without succumbing to apoptosis, cells harboring activated oncogenes augment their antioxidant capacity by increasing transactivation of genes encoding GSH- and TXN-dependent enzymes along with antioxidant/detoxification enzymes (1). Often this readjustment of redox entails loss or blunting of repression of NRF2 by KEAP1 and induction of NRF2 target genes. In addition, HIF-1α increases expression of key metabolic proteins, such as GLUT1, HK2, and MCT4. Also, NF-κB and TP53 probably contribute to this adaptive process, although in lesions harboring mutant TP53 the latter is unlikely. Treatment of animals with BSO, which inhibits synthesis of GSH, before initiation of carcinogenesis will stimulate apoptosis of premalignant cells (2). However, once carcinogenesis has been initiated, stimulation of apoptosis in malignant cells requires inhibition of both the GSH-based (by BSO) and the TXN-based (by sulfasalazine or auranofin) antioxidant systems. During the progression stage of tumorigenesis (B), EMT is triggered by a variety of environmental factors, including those that alter intracellular redox. In this regard, TGF-β signaling (1), growth factor signaling (2), and tumor-associated macrophages (TAMs) in the microenvironment (3) will produce ROS. Binding of TGF-β to its cognate receptor causes phosphorylation of SMAD2/3 (4) and induction of NOX4 gene expression (5), which results in production of H2O2 at the ER (6). NOX4-generated ROS within the tumor cell is augmented by growth factor signaling causing phosphorylation and activation of NOX1 and production of O2●– at the plasma membrane (7), with increased ROS increasing processing of latent TGF-β (8). The increases in ROS from TGF-β and growth factor signaling, along with those generated by TAMs, activate TP53, which combines with SMAD proteins to induce transcription of genes encoding the EMT-TFs SNAIL and TWIST (9). Similarly, increased ROS levels activate AP-1 (10) and HIF-1α (11) and induce SNAIL and TWIST, whereas the activation by ROS of NF-κB (12) leads to induction of genes encoding SNAIL, TWIST, SLUG, ZEB1, and ZEB2. Together, SNAIL, TWIST, SLUG, ZEB1, and ZEB2 positively control expression of mesenchymal-associated genes and negatively control expression of epithelial-associated genes. ROS also activate HSF1 (13), whereas its downregulation decreases TGF-β-mediated expression of SNAIL and SLUG and inhibits EMT, although the precise mechanism(s) is not understood.