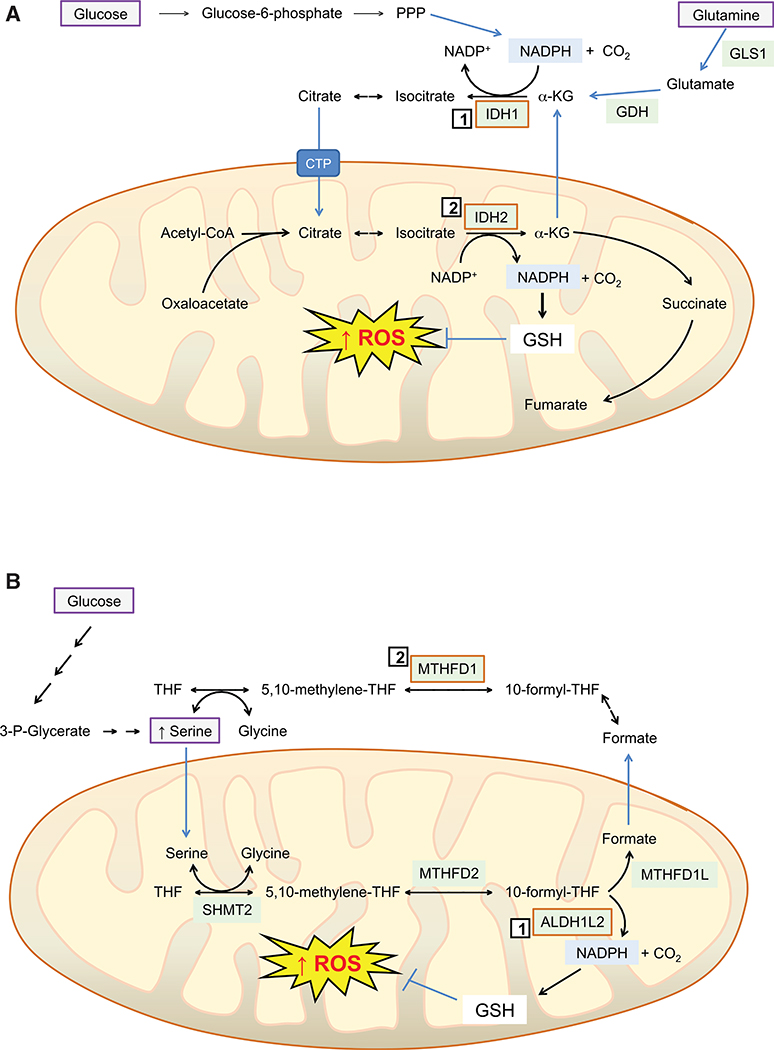
Figure 6. Reductive Glutamine Metabolism and Serine-Driven Folate Metabolism Suppresses Mitochondrial ROS Accumulation to Support Anchorage-Independent Growth and/or Metastatic Disease.
(A) The increase in mitochondrial ROS that occurs when tumor cells are grown as spheroids can be mitigated by the concerted actions of IDH1 (1) and IDH2 (2), located in the cytoplasm and mitochondrion, respectively. Specifically, within the cytoplasm, IDH1 catalyzes the reductive carboxylation of α-ketoglutarate (α-KG), obtained from glutamine (by the sequential actions of GLS1 and GDH, see top right of cartoon), utilizing NADPH provided by the pentose phosphate pathway (PPP) (see top left and center of cartoon), to provide a supply of isocitrate and citrate. In turn, citrate in the cytoplasm is transferred to the mitochondrion via the citrate transporter protein (CPT, SLC25A1), before it is utilized by IDH2 to produce α-KG and NADPH. The latter is required to reduce GSSG and maintain high GSH levels, which ensures mitochondrial ROS levels are restrained and anoikis averted. Thus, IDH1 and IDH2 activities in the different subcellular compartments enable NADPH generated by the PPP, along with high glutamate/glutamine levels, to drive reductive carboxylation of α-KG in the cytoplasm, and so transfer reducing equivalents from the cytoplasm to the mitochondrion.
(B) The 10-formyl-tetrahydrofolate (THF) pathway represents a major source of NADPH for a variety of cell lines grown under in vitro cell culture conditions. For melanoma tumor cells to survive the relatively high O2 levels in the bloodstream and then colonize the liver, they increase production of NADPH by augmenting ALDH1L2 protein levels and maintaining levels of MTHFD1; ALDH1L2 (1) and MTHFD1 (2) are located in the mitochondrion and cytoplasm, respectively. The cartoon depicts how high de novo synthesis of serine, derived from glucose and 3-phosphoglycerate (top left-hand side), followed by transport into the mitochondrion, allows donation of a methyl group to THF, yielding 5,10-methylene-THF through a serine hydroxymethyl transferase (SHMT) 2-catalyzed reaction. In turn, 5,10-methylene-THF within the mitochondrion is converted to 10-formyl-THF by MTHFD2, which can in turn be utilized by ALDH1L2 to form NADPH (bottom right-hand side), which maintains GSH levels and prevents excess ROS from accumulating. Alternatively, 10-formyl-THF can be used by MTHFD1L to generate formate, which when transported out of the mitochondrion can be used in reversible MTHFD1-catalyzed reactions to generate 5,10-methylene-THF.