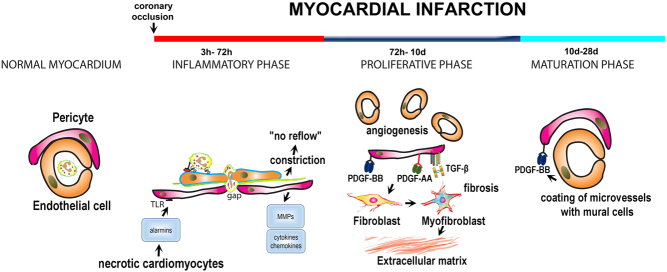
Figure 2.
The role of endogenous pericytes in myocardial infarction. The adult mammalian heart lacks regenerative capacity and heals through formation of a collagen-based scar. The reparative response can be divided into three distinct, but overlapping phases: the inflammatory phase, the proliferative phase and the maturation phase. The normal myocardium contains a large population of pericytes with peri-endothelial location. During the inflammatory phase, release of alarmins from dying cells may activate TLR-mediated pathways in pericytes, stimulating cytokine and chemokine secretion. Moreover, pericytes may produce matrix metalloproteinases, thus degrading the microvascular basement membrane and stimulating an angiogenic response. Formation of gaps between pericytes in the pro-inflammatory environment of the infarct may be required for leukocyte extravasation. Pericytes also regulate microvascular function. Constriction of pericytes in the ischemic and reperfused myocardium has been implicated in the pathogenesis of ‘no-reflow’. During the proliferative phase, pericytes activated by growth factors may regulate angiogenesis and fibrosis. To what extent pericyte to fibroblast conversion contributes to the expansion of myofibroblasts in healing infarcts remains unclear. Pericytes may also produce angiogenic and fibrogenic growth factors. During the maturation phase infarct neovessels recruit mural cells (both pericytes and vascular smooth muscle cells) through PDGFRβ-dependent pathways. Acquisition of a mural cell coat may be important to stabilize the infarct vasculature and to protect the infarcted heart from sustained inflammation.

 This work is licensed under a
This work is licensed under a