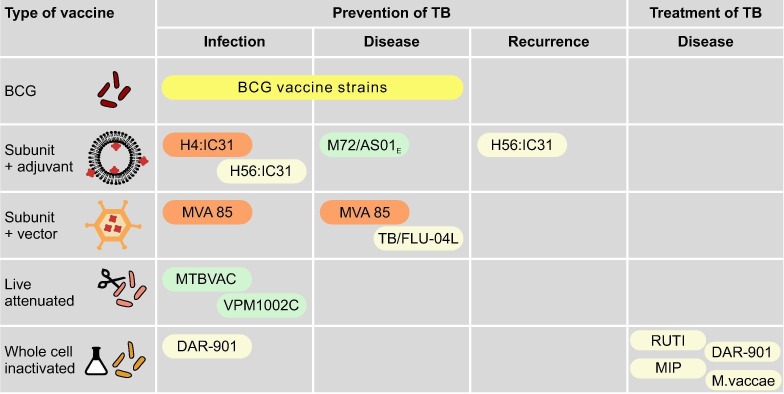
Fig. 2.
Novel TB vaccine strategies with example vaccines. Only vaccines that are in (or registered for) phase II or III trials are included. The colours around vaccines denote trials that have shown improved protection (green), no improvement (orange), or not discussed/no results available (yellow). H4:IC31: fusion protein of antigens 85B and TB10.4 with IC31 adjuvant H56:IC31: fusion protein of antigens 85B, ESAT-6 and Rv2660c with IC31 adjuvant M72/AS01E: fusion protein of antigen 32A and 39A with AS01E adjuvant MVA85: modified vaccinia Ankara 85A TB/FLU-04L: influenza A vectored vaccine expressing the antigens 85A and ESAT-6 MTBVAC: live attenuated M. tuberculosis strain (Euro-American lineage 4) with deletion mutations in the virulence genes phoP and fadD26 VPM1002C: live attenduated M. bovis BCG-Prague (rBCGΔureC:Hly) DAR-901: heat-killed, fragmented M. tuberculosis cultured under stress conditions MIP: heat-killed Mycobacterium indicus pranii a non-pathogenic, non-tuberculous mycobacterium approved in India as a leprosy vaccine M. vaccae: heat-killed Mycobacterium vaccae a non-pathogenic, non-tuberculous mycobacterium.